

Chem. J. Chinese Universities ›› 2016, Vol. 37 ›› Issue (3): 493.doi: 10.7503/cjcu20150702
• Organic Chemistry • Previous Articles Next Articles
SUN Baohou, ZHAO Yi, HAI Li, GUO Li*( ), WU Yong*(
), WU Yong*( )
)
Received:2015-09-08
Online:2016-03-10
Published:2015-12-26
Contact:
GUO Li,WU Yong
E-mail:guoli@scu.edu.cn;wyong@scu.edu.cn
Supported by:CLC Number:
TrendMD:
SUN Baohou, ZHAO Yi, HAI Li, GUO Li, WU Yong. Synthesis of Three Stereoisomers of 4-(3-Hydroxylbutoxy)-2-butanol†[J]. Chem. J. Chinese Universities, 2016, 37(3): 493.
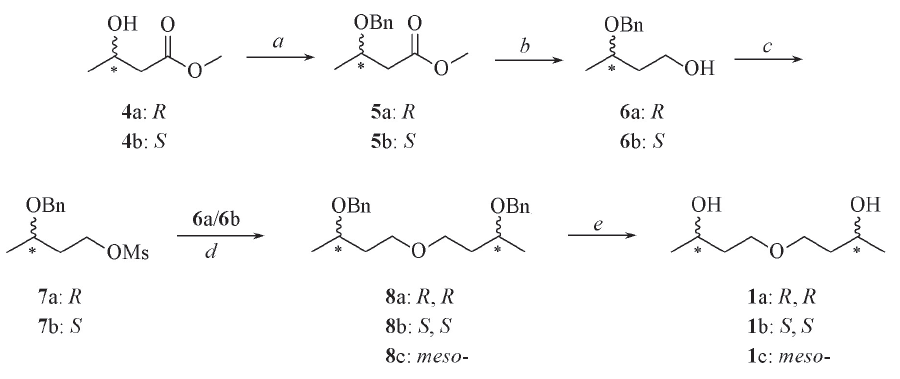
Scheme 1 Synthetic route of compounds 1a—1cReagents and conditions: a. benzyl trichloroacetimidate, TFA, CH2Cl2, r. t., 24 h; b. LiAlH4, THF, 0 ℃, 2 h; c. MsCl, Py, CH2Cl2, 0 ℃, 2 h; d. NaH, THF, 70 ℃, 5 h; e. H2, Pd/C, MeOH, 50 ℃, 2 h.
| Compd. | Appearance | Yield(%) | [α (c 1.0, CHCl3) | Elemental analysis(%, calcd.) | ESI-HRMS, m/z [M+Na]+ | |
|---|---|---|---|---|---|---|
| C | H | |||||
| 8a | Colorless oil | 85 | +62.3 | 77.20(77.16) | 8.85(8.83) | 365.2092 |
| 8b | Colorless oil | 86 | -61.1 | 77.18(77.16) | 8.86(8.83) | 365.2093 |
| 8c | Colorless oil | 83 | 0 | 77.18(77.16) | 8.80(8.83) | 365.2091 |
| 1a | Colorless oil | 95 | +10.9 | 59.21(59.23) | 11.15(11.18) | 185.1153 |
| 1b | Colorless oil | 96 | -10.0 | 59.26(59.23) | 11.19(11.18) | 185.1156 |
| 1c | Colorless oil | 95 | 0 | 59.25(59.23) | 11.20(11.18) | 185.1153 |
Table 1 Appearance, yields, optical rotation, elemental analysis and MS data of compounds 8a—8c and 1a—1c
| Compd. | Appearance | Yield(%) | [α (c 1.0, CHCl3) | Elemental analysis(%, calcd.) | ESI-HRMS, m/z [M+Na]+ | |
|---|---|---|---|---|---|---|
| C | H | |||||
| 8a | Colorless oil | 85 | +62.3 | 77.20(77.16) | 8.85(8.83) | 365.2092 |
| 8b | Colorless oil | 86 | -61.1 | 77.18(77.16) | 8.86(8.83) | 365.2093 |
| 8c | Colorless oil | 83 | 0 | 77.18(77.16) | 8.80(8.83) | 365.2091 |
| 1a | Colorless oil | 95 | +10.9 | 59.21(59.23) | 11.15(11.18) | 185.1153 |
| 1b | Colorless oil | 96 | -10.0 | 59.26(59.23) | 11.19(11.18) | 185.1156 |
| 1c | Colorless oil | 95 | 0 | 59.25(59.23) | 11.20(11.18) | 185.1153 |
| Compd. | 1H NMR(400 MHz, CDCl3), δ | 13C NMR(100 MHz, CDCl3), δ |
|---|---|---|
| 8a | 1.20(d, 6H, J=6.4 Hz), 1.66—1.74(m, 2H), 1.79—1.87(m, 2H), 3.42—3.55(m, 4H), 3.64—3.70(m, 2H), 4.50(ABq, 4H, J=11.6 Hz), 7.27—7.33(m, 10H) | 19.8(2C), 36.9(2C), 67.5(2C), 70.4(2C), 72.1(2C), 127.4(2C), 127.6(4C), 128.3(4C), 138.9(2C) |
| 8b | 1.20(d, 3H, J=6.0 Hz), 1.23(d, 3H, J=6.0 Hz), 1.66—1.90(m, 4H), 3.42—3.55(m, 4H), 3.61—3.68(m, 2H), 4.50(ABq, 4H, J=11.6 Hz), 7.25—7.33(m, 10H) | |
| 8c | 1.20(d, 3H, J=6.4 Hz), 1.21(d, 3H, J=6.4 Hz), 1.66—1.74(m, 2H), 1.80—1.88(m, 2H), 3.44—3.54(m, 4H), 3.63—3.71(m, 2H), 4.50(ABq, 4H, J=11.6 Hz), 7.25—7.25(m, 10H) | 19.8(2C), 36.9(2C), 67.5(2C), 70.4(2C), 72.2(2C), 127.3(2C), 127.6(4C), 128.2(4C), 138.9(2C) |
| 1a | 1.21(d, 6H, J=6.6 Hz), 1.70—1.73(m, 4H), 3.16(br, 2H), 3.57—3.60(m, 2H), 3.66—3.69(m, 2H), 3.96—4.01(m, 2H) | 23.3(2C), 37.9(2C), 67.1(2C), 69.5(2C) |
| 1b | 1.18(d, 6H, J=6.0 Hz), 1.67—1.71(m, 4H), 3.48(br, 2H), 3.53—3.67(m, 4H), 3.98—3.94(m, 2H) | |
| 1c | 1.17(d, 6H, J=6.4 Hz), 1.65—1.70(m, 4H), 3.52—3.66(m, 4H), 3.87(br, 2H), 3.92—4.00(m, 2H) | 23.3(2C), 37.9(2C), 66.8(2C), 69.2(2C) |
Table 2 1H NMR and 13C NMR data of compounds 8a—8c and 1a—1c
| Compd. | 1H NMR(400 MHz, CDCl3), δ | 13C NMR(100 MHz, CDCl3), δ |
|---|---|---|
| 8a | 1.20(d, 6H, J=6.4 Hz), 1.66—1.74(m, 2H), 1.79—1.87(m, 2H), 3.42—3.55(m, 4H), 3.64—3.70(m, 2H), 4.50(ABq, 4H, J=11.6 Hz), 7.27—7.33(m, 10H) | 19.8(2C), 36.9(2C), 67.5(2C), 70.4(2C), 72.1(2C), 127.4(2C), 127.6(4C), 128.3(4C), 138.9(2C) |
| 8b | 1.20(d, 3H, J=6.0 Hz), 1.23(d, 3H, J=6.0 Hz), 1.66—1.90(m, 4H), 3.42—3.55(m, 4H), 3.61—3.68(m, 2H), 4.50(ABq, 4H, J=11.6 Hz), 7.25—7.33(m, 10H) | |
| 8c | 1.20(d, 3H, J=6.4 Hz), 1.21(d, 3H, J=6.4 Hz), 1.66—1.74(m, 2H), 1.80—1.88(m, 2H), 3.44—3.54(m, 4H), 3.63—3.71(m, 2H), 4.50(ABq, 4H, J=11.6 Hz), 7.25—7.25(m, 10H) | 19.8(2C), 36.9(2C), 67.5(2C), 70.4(2C), 72.2(2C), 127.3(2C), 127.6(4C), 128.2(4C), 138.9(2C) |
| 1a | 1.21(d, 6H, J=6.6 Hz), 1.70—1.73(m, 4H), 3.16(br, 2H), 3.57—3.60(m, 2H), 3.66—3.69(m, 2H), 3.96—4.01(m, 2H) | 23.3(2C), 37.9(2C), 67.1(2C), 69.5(2C) |
| 1b | 1.18(d, 6H, J=6.0 Hz), 1.67—1.71(m, 4H), 3.48(br, 2H), 3.53—3.67(m, 4H), 3.98—3.94(m, 2H) | |
| 1c | 1.17(d, 6H, J=6.4 Hz), 1.65—1.70(m, 4H), 3.52—3.66(m, 4H), 3.87(br, 2H), 3.92—4.00(m, 2H) | 23.3(2C), 37.9(2C), 66.8(2C), 69.2(2C) |
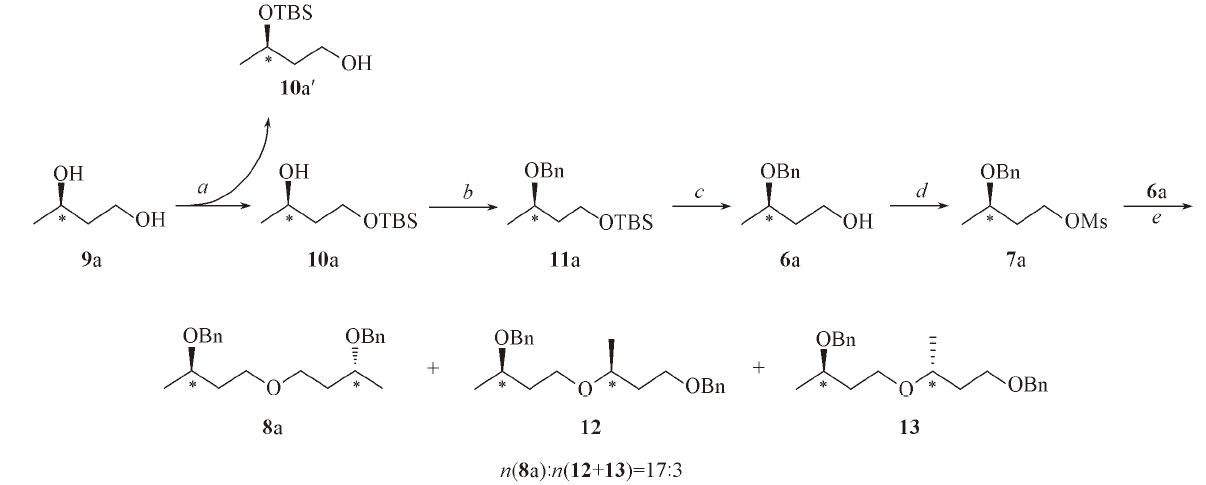
Scheme 2 Synthetic route of compound 8aReagents and conditions: a. imidazole, TBSCl, DMAP, DMF, r. t., 24 h; b. NaH, BnBr, THF, r. t., 10 h; c. TBAF, THF, 60 ℃, 4 h; d. MsCl, Py, CH2Cl2, 0 ℃, 2 h; e. NaH, THF, 70 ℃, 5 h.
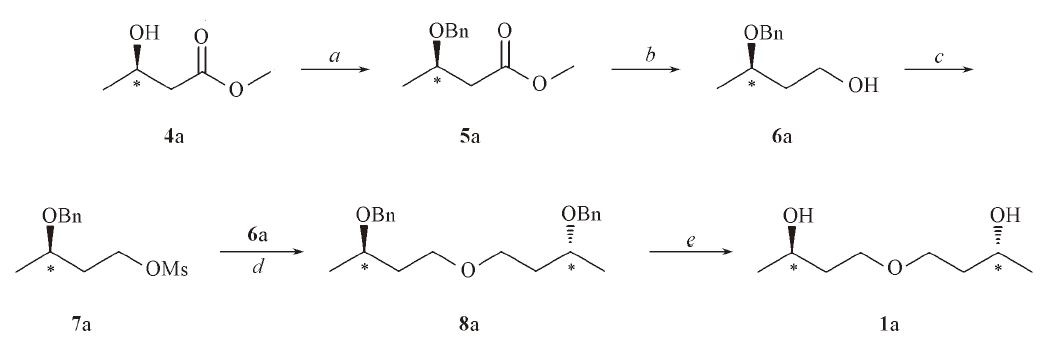
Scheme 2 Scheme 3 Synthetic route of compound 1aReagents and conditions: a. benzyl trichloroacetimidate, TFA, CH2Cl2, r. t., 24 h; b. LiAlH4, THF, 0 ℃, 2 h; c. MsCl, Py, CH2Cl2, 0 ℃, 2 h; d. NaH, THF, 70 ℃, 5 h; e. H2, Pd/C, MeOH, 50 ℃, 2 h.
| Entry* | Catalyst | n(Cat.)∶n(4a) | Solvent | Temperature/℃ | Time/h | Yield(%) |
|---|---|---|---|---|---|---|
| 1 | Et3N | 8∶1 | THF | r. t. | 72 | 0 |
| 2 | Imidazole | 8∶1 | THF | r. t. | 72 | 0 |
| 3 | NaH | 1.5∶1 | THF | r. t. | 72 | 0 |
| 4 | Ag2O | 8∶1 | THF | r. t. | 72 | 55 |
| 5 | Ag2O | 8∶1 | DMF | r. t. | 72 | 48 |
| 6 | Ag2O | 8∶1 | CH2Cl2 | r. t. | 72 | 61 |
| 7 | Ag2O | 8∶1 | EA | r. t. | 72 | 70 |
| 8 | Ag2O | 8∶1 | EA | r. t. | 36 | 57 |
| 9 | Ag2O | 8∶1 | EA | r. t. | 24 | 50 |
| 10 | CF3SO3H | 0.1∶1 | EA | r. t. | 24 | 59 |
| 11 | CF3SO3H | 0.1∶1 | DMF | r. t. | 24 | 45 |
| 12 | CF3SO3H | 0.1∶1 | THF | r. t. | 24 | 66 |
| 13 | CF3SO3H | 0.1∶1 | CH2Cl2 | r. t. | 24 | 85 |
| 14 | CF3SO3H | 0.1∶1 | CH2Cl2 | 0 | 24 | 58 |
| 15 | CF3SO3H | 0.1∶1 | CH2Cl2 | 40 | 24 | 73 |
Table 3 Synthetic reaction conditions of compound 5a
| Entry* | Catalyst | n(Cat.)∶n(4a) | Solvent | Temperature/℃ | Time/h | Yield(%) |
|---|---|---|---|---|---|---|
| 1 | Et3N | 8∶1 | THF | r. t. | 72 | 0 |
| 2 | Imidazole | 8∶1 | THF | r. t. | 72 | 0 |
| 3 | NaH | 1.5∶1 | THF | r. t. | 72 | 0 |
| 4 | Ag2O | 8∶1 | THF | r. t. | 72 | 55 |
| 5 | Ag2O | 8∶1 | DMF | r. t. | 72 | 48 |
| 6 | Ag2O | 8∶1 | CH2Cl2 | r. t. | 72 | 61 |
| 7 | Ag2O | 8∶1 | EA | r. t. | 72 | 70 |
| 8 | Ag2O | 8∶1 | EA | r. t. | 36 | 57 |
| 9 | Ag2O | 8∶1 | EA | r. t. | 24 | 50 |
| 10 | CF3SO3H | 0.1∶1 | EA | r. t. | 24 | 59 |
| 11 | CF3SO3H | 0.1∶1 | DMF | r. t. | 24 | 45 |
| 12 | CF3SO3H | 0.1∶1 | THF | r. t. | 24 | 66 |
| 13 | CF3SO3H | 0.1∶1 | CH2Cl2 | r. t. | 24 | 85 |
| 14 | CF3SO3H | 0.1∶1 | CH2Cl2 | 0 | 24 | 58 |
| 15 | CF3SO3H | 0.1∶1 | CH2Cl2 | 40 | 24 | 73 |
| [1] | Farooq M. O., Main A., Saeed B., Ikram A., Qasmi S. A., J. Ayub Med. Coll. Abbottabad,2014, 26(4), 543—547 |
| [2] | Chen L. L., Du R. M., Chen S. Y., Hebei Med., 2003, 9(8), 711—712 |
| (陈丽丽, 杜瑞明, 陈少逸. 河北医学, 2003,9(8), 711—712) | |
| [3] | Corbic M., Dumont M., de Couët G., Erlinger S., J. Pharmacol. Exp. Ther., 1982, 221(3), 769—774 |
| [4] | Zhu T. Y., Yuan J. Y., Xie B. G., Li Y. W., Chem. Reagents, 2015, 37(2), 168—170 |
| (朱太勇, 袁建勇, 谢宝刚, 李雁武. 化学试剂, 2015, 37(2), 168—170) | |
| [5] | Liu C., Guan X., Chin. Med. J. Metallurgical Industry,2008, 25(5), 624 |
| (刘畅, 官鑫. 中国冶金工业医学杂志, 2008, 25(5), 624) | |
| [6] | Missale G., Camarri E., Fici F., Agosti A., Di Murro R., Int. J. Clin. Pharmacol. Ther., 1981, 19(6), 273—274 |
| [7] | Staccioli S., Altamura M., Giorgi G., J. Mass Spectrom., 2009, 44(7), 1081—1086 |
| [8] | Marom H., Pogodin S., Agranat I., Chirality,2014, 26(4), 214—227 |
| [9] | Bulca S., Bayramgurler D., Demirsoy E. O., Yavuz M., Akturk A. S., Bilen N., Kiran R., J. Dermatol. Treat., 2013, 24(6), 473—476 |
| [10] | Yuan B., Li L., Fu Y., Jin Y., Guo L. X., Xu H. Y., J. Chromatogr. B, 2012, 911, 27—33 |
| [11] | Yue Q. M., Zhao Y., Sun B. H., Hai L., Guo L., Wu Y., Chin. J. Chem., 2015, 33, 1145—1152 |
| [12] | Zhao Y., Ma H. P., Fu Z. J., Zhang G. Y., Chem. Res. Chinese Universities, 2014, 30(6), 915—918 |
| [13] | Nie G. Z., Li L. S., Cheng B. P., Zhou R. D., Zhang H. F., Chem. J. Chinese Universities, 2014, 35(7), 1414—1422 |
| (聂桂珍, 李来生, 程彪平, 周仁丹, 张宏福. 高等学校化学学报, 2014, 35(7), 1414—1422) | |
| [14] | Tanasova M., Anyika M., Borhan B., Angew. Chem. Int. Ed., 2015, 54(14), 4274—4278 |
| [15] | Dong X., Li Y., Gou X. F., Zhao J. L., Hua C. W., Chem. Res. Chinese Universities, 2014, 30(3), 383—386 |
| [16] | Takahashi S., Souma K., Hashimoto R., Koshino H., Nakata T., J. Org. Chem., 2004, 69(13), 4509—4515 |
| [17] | Huang P. Q., Lan H. Q., Zheng X., Roan Y. P., J. Org. Chem., 2004, 69(11), 3964—3967 |
| [18] | Shen X., Neumann C. N., Kleinlein C., Angew. Chem. Int. Ed., 2015, 127(19), 5754—5757 |
| [19] | Evans P. A., Andrews W. J., Angew. Chem. Int. Ed., 2008, 47(29), 5426—5429 |
| [20] | Ren H., Wulff W. D., Org. Lett., 2013, 15(2), 242—245 |
| [1] | CHEN Fen-Er, LING Xiu-Hong, LÜ Yin-Xiang, ZHANG Xiao-Yue, PENG Xiao-Hua . Studies on the Asymmetry Total Synthesis of d-Biotin(Ⅱ) [J]. Chem. J. Chinese Universities, 2001, 22(7): 1141. |
| [2] | HUANG Jun-Min, CHEN Ru-Yu . Studies of Quinazolone Analogues Containing Phosphorus and Their Reaction with Aldehydes [J]. Chem. J. Chinese Universities, 2000, 21(10): 1510. |
| [3] | ZOU Kun, ZHAO Yu-Ying, WANG Bin, LI De-Yu, CAI Shao-Qing, ZHANG Ru-Yi . StructuraL Identification of Two Diastereoisomeric Saponins from Albizia Julibrissin [J]. Chem. J. Chinese Universities, 1999, 20(12): 1877. |
| [4] | ZHANG Cheng-Xiang, ZHANG Zhong-Biao, TANG Chu-Chi, CHEN Ru-Yu . Synthesis of Cyclic Glycerophospholipid Conjugates of Adenosine [J]. Chem. J. Chinese Universities, 1998, 19(6): 913. |
| [5] | SHAO Rui-Lian, YANG Guang-Fu, MIAO Wei-Shi, LI Guang-Ren . Studies on the Syntheses and Biological Activties of trans-Cyclothiophosphorylthioureas [J]. Chem. J. Chinese Universities, 1995, 16(3): 391. |
| [6] | Qiu Wen-yuan . A Study of the Topological Properties on the Molecular Cylinder and MÖbius Strip with Three-Rung and Four-Rung Ladder [J]. Chem. J. Chinese Universities, 1991, 12(11): 1532. |
| [7] | Chen Ru-yu, Liu Li-jian . A Research in the Asymmetric Reactions of Some Thiophosphoryldichlorides [J]. Chem. J. Chinese Universities, 1990, 11(12): 1372. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||