

Chem. J. Chinese Universities ›› 2015, Vol. 36 ›› Issue (9): 1779.doi: 10.7503/cjcu20150138
• Physical Chemistry • Previous Articles Next Articles
MENG Zhiyu, ZHANG Yin*( ), ZHAO Lili, ZHANG Hongxi, ZHAO Yongxiang*(
), ZHAO Lili, ZHANG Hongxi, ZHAO Yongxiang*( )
)
Received:2015-02-09
Online:2015-09-10
Published:2015-07-06
Contact:
ZHANG Yin,ZHAO Yongxiang
E-mail:sxuzhy@sxu.edu.cn;yxzhao@sxu.edu.cn
Supported by:CLC Number:
TrendMD:
MENG Zhiyu, ZHANG Yin, ZHAO Lili, ZHANG Hongxi, ZHAO Yongxiang. Liquid Phase Hydrogenation of Maleic Anhydride over Ni/TiO2 Catalysts with Different TiO2 Polymorphs†[J]. Chem. J. Chinese Universities, 2015, 36(9): 1779.
| Catalyst | SBET/(m2·g-1) | Pore volume/(cm3·g-1) | Average pore diameter/nm | Average Ni crystalline size/nm |
|---|---|---|---|---|
| A-TiO2 | 30 | 0.26 | 34.0 | |
| R-TiO2 | 30 | 0.23 | 30.3 | |
| 12.5%Ni/A-TiO2 | 22 | 0.20 | 35.0 | 13.5 |
| 12.5%Ni/R-TiO2 | 25 | 0.27 | 41.5 | 15.1 |
Table 1 Textural properties of catalysts
| Catalyst | SBET/(m2·g-1) | Pore volume/(cm3·g-1) | Average pore diameter/nm | Average Ni crystalline size/nm |
|---|---|---|---|---|
| A-TiO2 | 30 | 0.26 | 34.0 | |
| R-TiO2 | 30 | 0.23 | 30.3 | |
| 12.5%Ni/A-TiO2 | 22 | 0.20 | 35.0 | 13.5 |
| 12.5%Ni/R-TiO2 | 25 | 0.27 | 41.5 | 15.1 |
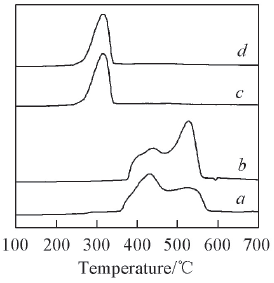
Fig.1 H2-TPR profiles of prepared Ni/TiO2 catalysts and mechanical mixture a. 12.5%Ni/A-TiO2; b. 12.5%Ni/R-TiO2; c. 12.5%Ni-A-TiO2; d. 12.5%Ni-R-TiO2.
| Catalyst | Actual consumption of H2/ (μmol·g-1) | Theoretical consumption of H2/ (μmol·g-1) |
|---|---|---|
| 12.5%Ni/A-TiO2 | 4446 | 2485 |
| 12.5%Ni/R-TiO2 | 4075 | 2485 |
| 12.5%Ni-A-Ti | 2927 | 2485 |
| 12.5%Ni-R-Ti | 2871 | 2485 |
Table 2 H2 consumption of the catalysts
| Catalyst | Actual consumption of H2/ (μmol·g-1) | Theoretical consumption of H2/ (μmol·g-1) |
|---|---|---|
| 12.5%Ni/A-TiO2 | 4446 | 2485 |
| 12.5%Ni/R-TiO2 | 4075 | 2485 |
| 12.5%Ni-A-Ti | 2927 | 2485 |
| 12.5%Ni-R-Ti | 2871 | 2485 |
| Catalyst | Eg/eV | nTi(Ⅲ) /[nTi(Ⅳ)+ nTi(Ⅲ)](%) | |
|---|---|---|---|
| Ti(Ⅲ | Ti(Ⅳ) | ||
| 12.5%Ni/A-TiO2 | 456.9 | 458.1 | 30.2 |
| 12.5%Ni/R-TiO2 | 457.0 | 458.2 | 14.6 |
Table 3 Fitting results of XPS data of 12.5%Ni/TiO2 catalysts
| Catalyst | Eg/eV | nTi(Ⅲ) /[nTi(Ⅳ)+ nTi(Ⅲ)](%) | |
|---|---|---|---|
| Ti(Ⅲ | Ti(Ⅳ) | ||
| 12.5%Ni/A-TiO2 | 456.9 | 458.1 | 30.2 |
| 12.5%Ni/R-TiO2 | 457.0 | 458.2 | 14.6 |
| Catalyst | Hydrogen pressure/MPa | Conversion of MA(%) | Selectivity(%) | TO | |
|---|---|---|---|---|---|
| GBL | SA | ||||
| 12.5%Ni/A-TiO2 | 3 | 99.9 | 45.2 | 54.8 | 0.98 |
| 4 | 100 | 51.5 | 48.5 | 1.12 | |
| 5 | 100 | 60.0 | 40.0 | 1.30 | |
| 12.5%Ni/R-TiO2 | 3 | 100 | 17.9 | 82.1 | 0.35 |
| 4 | 100 | 24.5 | 75.5 | 0.48 | |
| 5 | 100 | 32.9 | 67.1 | 0.64 | |
Table 4 Effect of hydrogen pressure on catalytic performance of catalysts for maleic anhydride hydrogenationa
| Catalyst | Hydrogen pressure/MPa | Conversion of MA(%) | Selectivity(%) | TO | |
|---|---|---|---|---|---|
| GBL | SA | ||||
| 12.5%Ni/A-TiO2 | 3 | 99.9 | 45.2 | 54.8 | 0.98 |
| 4 | 100 | 51.5 | 48.5 | 1.12 | |
| 5 | 100 | 60.0 | 40.0 | 1.30 | |
| 12.5%Ni/R-TiO2 | 3 | 100 | 17.9 | 82.1 | 0.35 |
| 4 | 100 | 24.5 | 75.5 | 0.48 | |
| 5 | 100 | 32.9 | 67.1 | 0.64 | |
| Catalyst | T/K | Conversion of MA(%) | Selectivity(%) | TO | |
|---|---|---|---|---|---|
| GBL | SA | ||||
| 12.5%Ni/A-TiO2 | 453 | 99.3 | 31.9 | 68.1 | 0.69 |
| 483 | 100 | 60.0 | 40.0 | 1.30 | |
| 513 | 100 | 62.8 | 37.2 | 1.36 | |
| 12.5%Ni/R-TiO2 | 453 | 100 | 9.3 | 90.7 | 0.18 |
| 483 | 100 | 32.9 | 67.1 | 0.64 | |
| 513 | 100 | 33.1 | 66.9 | 0.64 | |
Table 5 Effect of reaction temperature on catalytic performance of catalysts for maleic anhydride hydrogenationa
| Catalyst | T/K | Conversion of MA(%) | Selectivity(%) | TO | |
|---|---|---|---|---|---|
| GBL | SA | ||||
| 12.5%Ni/A-TiO2 | 453 | 99.3 | 31.9 | 68.1 | 0.69 |
| 483 | 100 | 60.0 | 40.0 | 1.30 | |
| 513 | 100 | 62.8 | 37.2 | 1.36 | |
| 12.5%Ni/R-TiO2 | 453 | 100 | 9.3 | 90.7 | 0.18 |
| 483 | 100 | 32.9 | 67.1 | 0.64 | |
| 513 | 100 | 33.1 | 66.9 | 0.64 | |
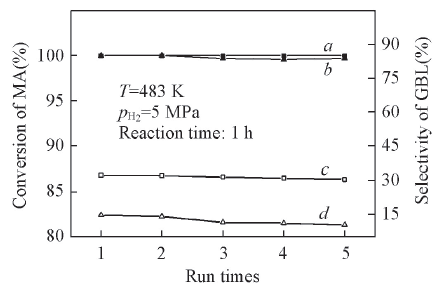
Fig.6 Effect of run times on catalytic performance of catalysts for MA conversion(a, b) and GBL selectivity(c, d)a, c. 12.5%Ni/A-TiO2; b, d. 12.5%Ni/R-TiO2.
| [1] | Jung S. M., Godard E., Jung S. Y., Park K. C., Choi J. U., Catal Today, 2003, 87(1—4), 171—177 |
| [2] | Hu T. J., Yin H. B., Zhang R. C., Wu H. X., Jiang T. S., Wada Y. J., Catal. Commun., 2007, 8(2), 193—199 |
| [3] | Harris N., Tuck M. W., Hydrocarbon Process, 1990, 69(5), 79—82 |
| [4] | Papageorgiou G. Z., Grigoriadou I., Andriotis E., Bikiaris D. N, Panayiotou C., Ind. Eng. Chem. Res., 2013, 52(34), 11948—11955 |
| [5] | Xu J., Sun K. P., Zhang L., Catal. Commun., 2005, 6(7), 462—465 |
| [6] | Xu J., Sun K. P., Zhang L., Catal. Lett., 2006, 107(1/2), 5—11 |
| [7] | Seong M. J., Eric G., Sang Y. J., Catal Today, 2003, 87(1—4), 171—177 |
| [8] | Zhao Y. X., Qing X. Q., Hou X. C., Acta. Phys. Chim. Sin., 2003, 19(5), 450—454 |
| (赵永祥, 秦晓琴, 侯希才. 物理化学学报, 2003, 19(5), 450—454) | |
| [9] | Regenhardt S. A., Trasarti A. F., Meyer C. I., Garetto T. F., Marchi A. J., Catal. Commun., 2013, 35, 59—63 |
| [10] | Hong U. G., Park H. W., Lee J., Hwang S., Song I. K., J. Ind. Eng. Chem., 2012, 18(1), 462—468 |
| [11] | Budroni G., Corma A., J. Catal., 2008, 257(2), 403—408 |
| [12] | Wang Q., Cheng H., Liu R., Hao J., Yu Y., Zhao F., Catal. Today, 2009, 148(3/4), 368—372 |
| [13] | Feng Y., Yin H., Wang A., Xie T., Jiang T., Appl. Catal. A: Gen., 2012, 425/426(1), 205—212 |
| [14] | Regenhardt S. A., Meyer C. I., Garetto T. F., Marchi A. J., Appl. Catal. A: Gen., 2012, 449, 81—87 |
| [15] | Guo S. F., Shi L., Catal. Today, 2013, 212, 137—141 |
| [16] | Meyer C. I., Regenhardt S. A., Bertone M. E., Marchi A. J., Garetto T. F., Catal. Lett., 2013, 143(10), 1067—1073 |
| [17] | Yang Y. P., Zhang Y., Gao C. G., Zhao Y. X., Chin. J. Catal., 2011, 32(11), 1768—1774 |
| (杨燕萍, 张因, 高春光, 赵永祥. 催化学报, 2011, 32(11), 1768—1774) | |
| [18] | Kong W., Zhang X. H., Zhang Q., Wang T. J., Ma L. L., Chen G. Y., Chem. J. Chinese Universities, 2013, 34(12), 2806—2813 |
| (孔韡, 张兴华, 张琦, 王铁军, 马隆龙, 陈冠益. 高等学校化学学报, 2013, 34(12), 2806—2813) | |
| [19] | Shen W. J., Okumura M., Matsumura Y., Haruta M., Appl. Catal. A: Gen., 2001, 213(2), 225—232 |
| [20] | Fang C., Chen Y. J., Mao H., Zhao J., Jiang Y. F., Zhao S. L., Ma J., Chem. J. Chinese Universities, 2015, 36(1), 124—130 |
| (方超, 陈亚君, 毛卉, 赵俊, 蒋云福, 赵仕林, 马骏. 高等学校化学学报, 2015, 36(1), 124—130) | |
| [21] | Xiong J., Chen J. X., Zhang J. Y., Catal. Commun., 2007, 8(3), 345—350 |
| [22] | Wang X. J., Qi Q. H., Chen L., Chem. J. Chinese Universities, 2014, 35(10), 2138—2145 |
| (王秀军, 齐秋红, 陈丽. 高等学校化学学报, 2014, 35(10), 2138—2145) | |
| [23] | Shah P., Bhange D. S., Deshpande A. S., Chem. Phys., 2009, 117, 399—407 |
| [24] | Widchaya R., Araya T., Ratchaneekorn W., Chem. Res. Chinese Universities, 2014, 30(1), 149—156 |
| [25] | Saadi A., Merabti R., Rassoul Z., Bettahar M. M., J. Mol. Catal., 2006, 253(1/2), 79—85 |
| [26] | Xie G. Q., Liu X. J., Tao L. P., Lu J. Q., Luo M. F., Li X. N., Chin. J. Catal., 2009, 30(6), 543—548 |
| (谢冠群, 刘西敬, 陶丽萍, 鲁继青, 罗孟飞, 李小年. 催化学报, 2009, 30(6), 543—548) | |
| [27] | Li Y. Z., Xu B. L., Fan Y. N., Feng N. Y., Qiu A. D., Miao J. W., He J., Yang H. P., Chen Y., J. Mol. Catal. A: Chem., 2004, 216, 107—114 |
| [28] | Yao N., Chen J. X., Zhang J. X., Zhang J. Y., Catal. Commun., 2008, 9(6), 1510—1516 |
| [29] | Ho S. W., Chu C. Y., Chen S. G., J. Catal., 1998, 178(1), 34—48 |
| [30] | Yang Q. Y., Zhu Y., Tian L., Pei Y., Qiao M. H., Fan K. N., Acta. Phys. Chim. Sin., 2009, 25(9), 1853—1860 |
| (杨秋芸, 朱渊, 田莉, 裴燕, 乔明华, 范康年. 物理化学学报, 2009, 25(9), 1853—1860) | |
| [31] | Li Y. Z., Fan Y. N., Yang H. P., Xu B. L., Feng L. Y., Yang M. F., Chen Y., Chem. Phys. Lett., 2003, 372(1/2), 160—165 |
| [32] | Pouilleau J., Devilliers D., Groult H., Marcus P., J. Mater. Sci., 1997, 47(3), 235—243. |
| [33] | Bradford M. C. J., Vannice M. A., J. Catal., 1998, 173(1), 157—171 |
| [34] | Huo W. T., Zhang C. L., Yuan H. J., Jia H. J., Ning C. L., Tang Y., Zhang Y., Luo J. H., Wang Z. L., Zhang W. X., J. Ind. Eng. Chem., 2014, 20(6), 4140—4145 |
| [35] | Wang Y., Wang F., Song Q., Xin Q., Xu S., Xu J., J. Am. Chem. Soc., 2013, 135(4), 1506—1521 |
| [36] | Englisch M., Jentys A., Lercher J. A., J. Catal., 1997, 166(1), 25—35 |
| [1] | WU Hao, WANG Changzhen, QIU Yuan, TIAN Yani, ZHAO Yongxiang. Effect of Steric Confinement Dimension on Metal Site Anti-carbon Deposition Ability of Ni-SiO2 Catalysts in CH4-CO2 Reforming [J]. Chem. J. Chinese Universities, 2020, 41(11): 2488. |
| [2] | GUO Fang, CHU Wei*, SHI Xin-Yu, ZHANG Xu. Effects of Plasma Introduction Mode on Ni/γ-Al2O3 Catalysts for CH4 Reforming with CO2 [J]. Chem. J. Chinese Universities, 2009, 30(4): 746. |
| [3] | LI Ji-Tao, CHEN Ming-Dan, YAN Qian-Gu, WAN Hui-Lin, TSAI K. R. . Development of Higher Stable Ni/MgO Catalyst for CO2 Reforming of Methane [J]. Chem. J. Chinese Universities, 2000, 21(9): 1445. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||