

Chem. J. Chinese Universities ›› 2015, Vol. 36 ›› Issue (3): 575.doi: 10.7503/cjcu20140932
• Polymer Chemistry • Previous Articles Next Articles
HE Xiaohui*( ), LIU Jingyin, CHEN Lu, ZHU Hongyu, WANG Juan
), LIU Jingyin, CHEN Lu, ZHU Hongyu, WANG Juan
Received:2014-10-22
Online:2015-03-10
Published:2015-01-23
Contact:
HE Xiaohui
E-mail:hexiaohui@ncu.edu.cn
Supported by:CLC Number:
TrendMD:
HE Xiaohui, LIU Jingyin, CHEN Lu, ZHU Hongyu, WANG Juan. Copolymerization of Norbornene and n-Butyl Methacrylate Catalyzed by Novel Ni(Ⅱ)(benzocyclohexan-ketoarylimino) 2 †[J]. Chem. J. Chinese Universities, 2015, 36(3): 575.
| Cat. | n(NB)/n(n-BMA) | Yield(%) | 10-5Activity/ (gpolymer·mo | Mw/ | Molar fraction of n-BMA in copolymersc(%) | |
|---|---|---|---|---|---|---|
| C1 | 100/0 | 79.20 | 2.98 | Insoluble | 0 | |
| C1 | 80/20 | 42.35 | 1.75 | 3.61×104 | 1.10 | 3.82 |
| C1 | 60/40 | 26.44 | 1.19 | 4.35 | ||
| C1 | 50/50 | 9.54 | 0.45 | 2.97×104 | 1.13 | 5.60 |
| C2 | 100/0 | 73.10 | 2.75 | Insoluble | 0 | |
| C2 | 80/20 | 41.88 | 1.73 | 3.11×104 | 1.14 | 0.33 |
| C2 | 60/40 | 23.79 | 1.07 | 0.68 | ||
| C2 | 50/50 | 9.17 | 0.43 | 3.31×104 | 1.07 | 1.20 |
Table 1 Copolymerizations of NB/n-BMA catalyzed by C1/B(C6F5)3 and C2/B(C6F5)3 systemsa
| Cat. | n(NB)/n(n-BMA) | Yield(%) | 10-5Activity/ (gpolymer·mo | Mw/ | Molar fraction of n-BMA in copolymersc(%) | |
|---|---|---|---|---|---|---|
| C1 | 100/0 | 79.20 | 2.98 | Insoluble | 0 | |
| C1 | 80/20 | 42.35 | 1.75 | 3.61×104 | 1.10 | 3.82 |
| C1 | 60/40 | 26.44 | 1.19 | 4.35 | ||
| C1 | 50/50 | 9.54 | 0.45 | 2.97×104 | 1.13 | 5.60 |
| C2 | 100/0 | 73.10 | 2.75 | Insoluble | 0 | |
| C2 | 80/20 | 41.88 | 1.73 | 3.11×104 | 1.14 | 0.33 |
| C2 | 60/40 | 23.79 | 1.07 | 0.68 | ||
| C2 | 50/50 | 9.17 | 0.43 | 3.31×104 | 1.07 | 1.20 |
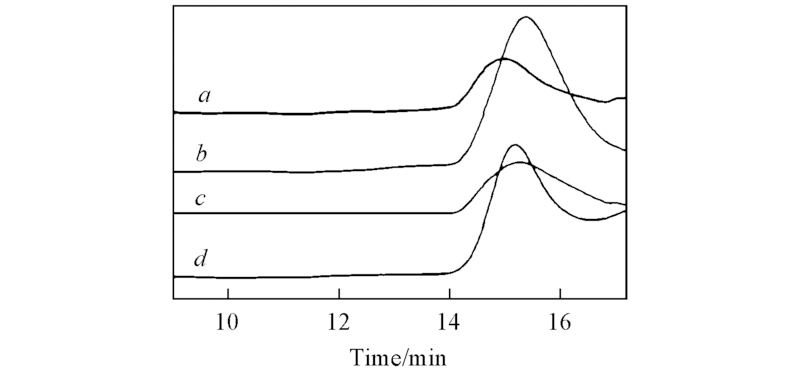
Fig.1 GPC curves for NB/n-BMA copolymers with Mw=3.61×104(a), Mw=2.97×104(b), Mw=3.11×104(c) and Mw=3.31×104(d) obtained by C1/B(C6F5)3(a, b) and C2/B(C6F5)3(c, d) catalytic systems
| Cat. | Molar fraction of n-BMA in the feed(%) | Molar fraction of n-BMA in copolymersa(%) | Yieldb(%) | Polymerization time/min |
|---|---|---|---|---|
| C1 | 50 | 5.60 | 9.54 | 30 |
| C1 | 60 | 7.27 | 7.69 | 30 |
| C1 | 70 | 11.15 | 2.95 | 25 |
| C1 | 80 | 22.60 | 1.45 | 25 |
| C1 | 90 | 27.30 | 0.51 | 40 |
| C2 | 50 | 1.20 | 9.17 | 30 |
| C2 | 60 | 2.25 | 4.42 | 30 |
| C2 | 70 | 2.69 | 2.36 | 25 |
| C2 | 80 | 3.79 | 1.38 | 25 |
| C2 | 90 | 6.54 | 0.48 | 40 |
Table 2 Copolymerization of NB/n-BMA catalyzed by C1/B(C6F5)3 and C2/B(C6F5)3 catalytic systems
| Cat. | Molar fraction of n-BMA in the feed(%) | Molar fraction of n-BMA in copolymersa(%) | Yieldb(%) | Polymerization time/min |
|---|---|---|---|---|
| C1 | 50 | 5.60 | 9.54 | 30 |
| C1 | 60 | 7.27 | 7.69 | 30 |
| C1 | 70 | 11.15 | 2.95 | 25 |
| C1 | 80 | 22.60 | 1.45 | 25 |
| C1 | 90 | 27.30 | 0.51 | 40 |
| C2 | 50 | 1.20 | 9.17 | 30 |
| C2 | 60 | 2.25 | 4.42 | 30 |
| C2 | 70 | 2.69 | 2.36 | 25 |
| C2 | 80 | 3.79 | 1.38 | 25 |
| C2 | 90 | 6.54 | 0.48 | 40 |
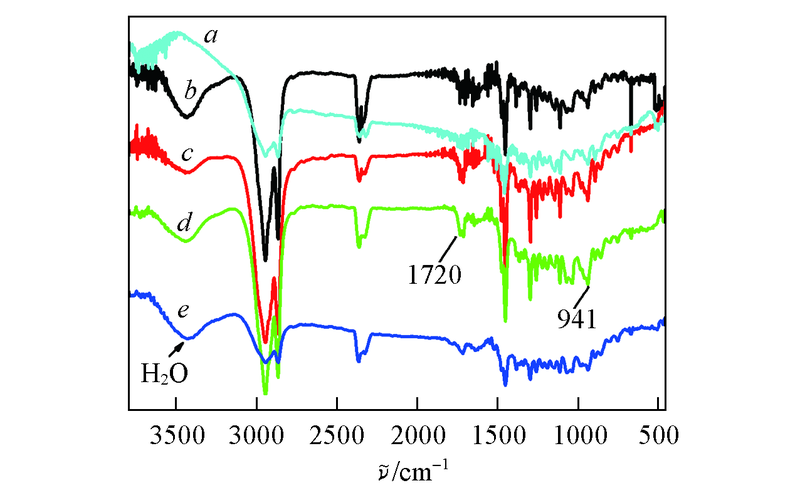
Fig.3 FTIR spectra of NB/n-BMA copolymers with 0(a), 3.82%(b), 5.60%(c), 0.33%(d), 1.20%(e) of n-BMA molar ratio obtained by C1/B(C6F5)3(a–c) and C2/B(C6F5)3(d, e) catalytic systems
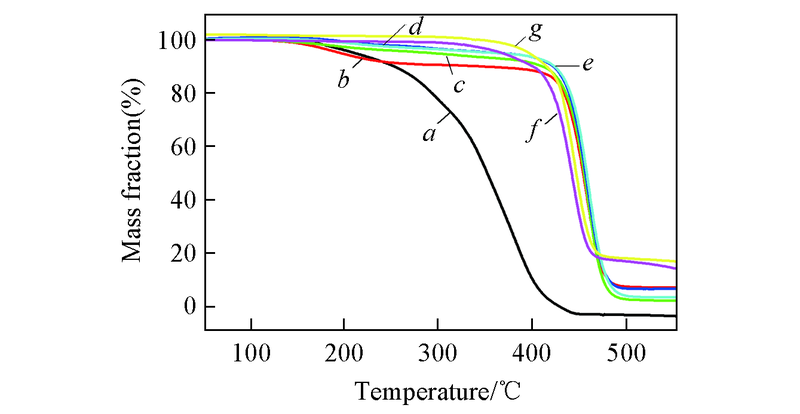
Fig.5 TGA curves of NB/n-BMA copolymers with 100%(a), 4.35%(b), 3.82%(c), 0(g) n-BMA molar ratio obtained by C1/B(C6F5)3 system and 0.33%(d), 0.68%(e), 0(f) of n-BMA molar ratio obtained by C2/B(C6F5)3 system
| [1] | Hong M., Cui L., Liu S., Li Y. S., Macromolecules,2012, 45, 5397—5402 |
| [2] | Zhong G. J., Su R., Zhang L. F., Wang K., Li Z. M., Fong H., Zhu L., Polymer,2012, 53, 4472—4480 |
| [3] | Matos J. M. E., Lima-Neto B. S., J. Mol. Catal. A: Chem., 2005, 240, 233—238 |
| [4] | Kong Y., Xu S. S., Song H. B., Wang B. Q., Organometallics,2012, 31, 5527—5532 |
| [5] | McCann M., Beaumont A. J., J. Mol. Catal. A: Chem., 1996, 108, 23—27 |
| [6] | McCann M., Beaumont A. J., J. Mol. Catal. A: Chem., 1996, 111, 251—255 |
| [7] | Kim J. G., Coates G. W., Macromolecules,2012, 45, 7878—7883 |
| [8] | Bencze L., Szalai G., Hamilton J. G., Rooney J. J., J. Mol. Catal. A: Chem., 1997, 115, 193—197 |
| [9] | Haselwander T. F. A., Heitz W., Krügel S. A., Wendorff J. H., Macromolecules,1997, 30, 5345—5351 |
| [10] | Song D. P., Mu H. L., Shi X. C., Li Y. G., Li Y. S., J. Polym. Sci. Part A: Polym. Chem., 2012, 50(3), 562—570 |
| [11] | Hao P., Song S. J., Xiao T. P. F, Li Y., Redshaw C., Sun W. H., Polyhedron,2013, 52, 1138—1144 |
| [12] | Berding J., Lutz M., Spek A. L, Bouwman E., Appl. Organomet. Chem., 2011, 25(1), 76—81 |
| [13] | Antonov A. A., Semikolenova N. V., Zakharov V. A., Zhang W. J., Wang Y. H., Sun W. H., Talsi E. P., Bryliakov K. P., Organometallics,2012, 31, 1143—1149 |
| [14] | Long J. M., Gao H. Y., Liu F. S., Song K. M., Hu H., Zhang L., Zhu F. M., Wu Q., Inorg. Chim. Acta,2009, 362, 3035—3042 |
| [15] | Cai Z. G., Harada R., Nakayama Y., Shiono T., Macromolecules,2010, 43, 4527—4531 |
| [16] | Huang Z. F., Gao H. Y., Wu Q., Chem. J. Chinese Universities,2010, 31(6), 1257—1262 |
| (黄增芳, 高海洋, 伍青. 高等学校化学学报, 2010, 31(6), 1257—1262) | |
| [17] | Li X., Ni X. F., Shen Z. Q., Chem. J. Chinese Universities,2012, 33(5), 1095—1099 |
| (李欣, 倪旭峰, 沈之荃. 高等学校化学学报, 2012, 33(5), 1095—1099) | |
| [18] | Zhang H., Zhang H. X., Cai X. P., Hao X. F., Zhang X. Q., Chem. J. Chinese Universities,2012, 33(12), 2784—2788 |
| (张浩, 张贺新, 蔡小平, 郝秀峰, 张学全. 高等学校化学学报, 2012, 33(12), 2784—2788) | |
| [19] | Chen L., Zhong Z. C., Chen C., He X. H., Chen Y. W., Journal of Organometallic Chemistry,2014, 752, 100—108 |
| [20] | Kelen T., Tüdõs F., J. Macromol. Sci. Pure Appl. Chem., 1975, 9(1), 1—27 |
| [21] | Wendt R. A., Angermund K., Jensen V., Thiel W., Fink G., Macromol. Chem. Phys., 2004, 205(3), 308—318 |
| [1] | MENG Jiafeng, NI Xufeng, ZHENG Hao, SHEN Zhiquan. Copolymerization of Norbornene and 1-Octene Catalyzed by Bis(phenoxy-imine) Titanium Complex† [J]. Chem. J. Chinese Universities, 2018, 39(8): 1853. |
| [2] | WANG Jun, HUO Hongliang, LI Cuiqin, MA Lili, SHI Weiguang, CHEN Shuai. Synthesis of Novel Dendritic Bridging Nickel Complex for Oligomerization of Olefins† [J]. Chem. J. Chinese Universities, 2015, 36(9): 1813. |
| [3] | WANG Jun, YANG Guang, LI Cuiqin, SHI Weiguang. Synthesis and Ethylene Oligomerization Catalytic Activities of Hyperhranched Salicylaldehyde-imine Nickel Complex [J]. Chem. J. Chinese Universities, 2014, 35(7): 1536. |
| [4] | ZHANG Danfeng, LI Sen, YU Wen, MENG Jiangang. Polymerization of Methyl Methacrylate with α-Diimine Nickel(Ⅱ)† [J]. Chem. J. Chinese Universities, 2014, 35(7): 1559. |
| [5] | ZHANG Dan-Feng, FAN Shuai, FU Yan, LI Sen. Synthesis of Branched Polyethylene and Characterization of Their Structures and Properties [J]. Chem. J. Chinese Universities, 2013, 34(8): 2005. |
| [6] | QIAN Tao, WANG Juan-Juan, ZHANG Qing-Hua, ZHAN Xiao-Li, CHEN Feng-Qiu. Synthesis and Characterization of Gradient Copolymer Composed of [N-Methyl-perfluorohexane-1-sulfonamide] Ethyl Acrylate and MMA [J]. Chem. J. Chinese Universities, 2013, 34(3): 703. |
| [7] | ZHANG Hao, ZHANG He-Xin, CAI Xiao-Ping, HAO Xiu-Feng, ZHANG Xue-Quan. Synthesis of 8-Anilino-1-naphthalenesulfonic Titanium Complex and Ethylene-norbornene Copolymerization [J]. Chem. J. Chinese Universities, 2012, 33(12): 2784. |
| [8] | LI Xin, NI Xu-Feng, SHEN Zhi-Quan. Copolymerization of Norbornene with Isoprene Catalyzed by TiCl4/Al(#em/em#-Bu)3 System [J]. Chem. J. Chinese Universities, 2012, 33(05): 1095. |
| [9] | WANG Yuan-Yuan, ZHU Fang-Ming, LIN Shang-An*. Norbornene Polymerization Catalyzed by Bispyrazolylimineylimine Nickel(Ⅱ)/MAO Catalytic Systems [J]. Chem. J. Chinese Universities, 2008, 29(8): 1684. |
| [10] | DU Chuang1, WANG Chun-Yu2, ZHANG Guo1, TANG Jun2*. Molecular Weight and Molecular Weight Distribution Control of Ring-opening Metathesis Polymerization of Norbornene [J]. Chem. J. Chinese Universities, 2007, 28(10): 2018. |
| [11] | FAN Rui-Qing, ZHU Dong-Sheng, MU Ying, LI Guang-Hua, FENG Shou-Hua. Synthesis, Characterization, Crystal Structures of Bis(imino)pyridyl Nickel Complexes and Their Catalytic Performance for Ethylene Polymerization [J]. Chem. J. Chinese Universities, 2005, 26(7): 1215. |
| [12] | DU Miao, WENG Zhi-Xue, SHAN Guo-Rong, HUANG Zhi-Ming, PAN Zu-Ren . Reactivity Ratios and Copolymer Composition of Vinyl Chloride and N-Substituted Maleimide Copolymerization [J]. Chem. J. Chinese Universities, 2004, 25(10): 1962. |
| [13] | DU Zhe-Wei, LIAN Yan-Qing, WANG Xiao-Gong, ZHOU Qi-Xiang, LIU De-Shan . Studies on Sequence Distribution of Copolyureas and Relationship of Diamines Reactivity Ratio [J]. Chem. J. Chinese Universities, 2001, 22(9): 1587. |
| [14] | LU Ying-Ying, WU Qing, LU Ze-Jian . Studies on Polymerization of Norbornene with Titanocene/MAO Catalyst System [J]. Chem. J. Chinese Universities, 2001, 22(1): 160. |
| [15] | ZHAO You-Liang, LI Hua-Ming, LIU Peng-Sheng . Studies on Microemulsion Copolymerization of N-Butyl Maleimide and Styrene [J]. Chem. J. Chinese Universities, 2000, 21(9): 1477. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||