

Chem. J. Chinese Universities ›› 2014, Vol. 35 ›› Issue (3): 602.doi: 10.7503/cjcu20130795
• Physical Chemistry • Previous Articles Next Articles
LI Qiaoxia1, MAO Hongmin1, ZHU Pingping1, CAO Xiaolu1, LU Tianhong2, XU Qunjie1,*( )
)
Received:2013-08-16
Online:2014-03-10
Published:2019-08-01
Contact:
XU Qunjie
E-mail:xuqunjie@shiep.edu.cn
Supported by:CLC Number:
TrendMD:
LI Qiaoxia, MAO Hongmin, ZHU Pingping, CAO Xiaolu, LU Tianhong, XU Qunjie. Electrocatalytic Performance of Pd-Sn/C Catalyst Prepared with Different Complexants for Ethanol Oxidation in Alkaline Solution†[J]. Chem. J. Chinese Universities, 2014, 35(3): 602.
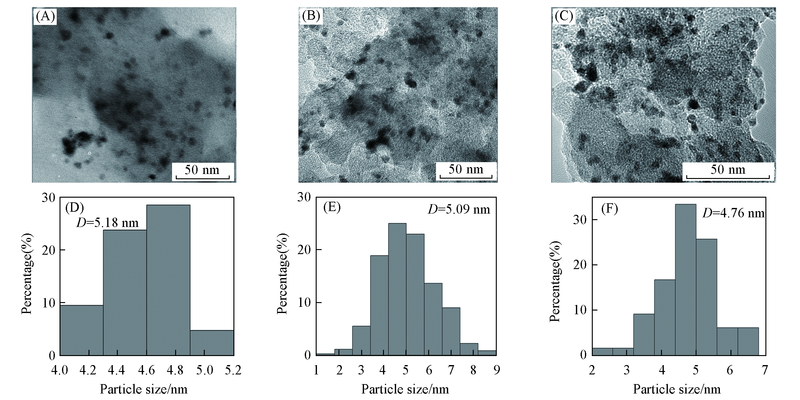
Fig.1 TEM images(A—C) and histogram of particle size distribution(D—F) of Pd-Sn/C-ammonia(A, D), Pd-Sn/C-CyDTA(B, E) and Pd-Sn/C-trisodium citrate(C, F) catalysts
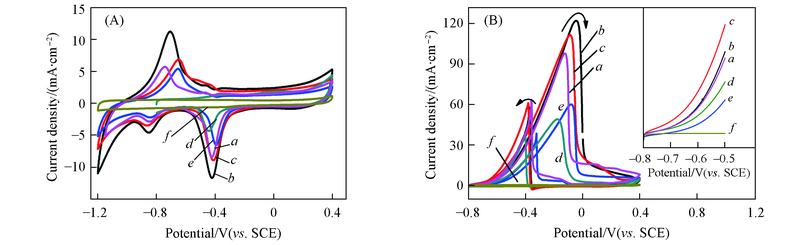
Fig.5 Cyclic voltammograms of Pd-Sn/C-ammonia(a), Pd-Sn/C-CyDTA(b), Pd-Sn/C-trisodium citrate(c), Pd/C(d), Pd-Sn/C(e), Sn/C catalysts(f) in 1.0 mol/L KOH(A) or 1.0 mol/L KOH+1.0 mol/L C2H5OH(B) at a scan rate of 50 mV/s
| Catalyst | Crystallite size/nm | EASA/(m2·g-1) | Eop/V | Ep/V | ip/(mA·cm-2) | |
|---|---|---|---|---|---|---|
| XRD | TEM | |||||
| Pd-Sn/C-CyDTA | 4.5 | 5.09 | 297.62 | -0.632 | -0.046 | 122.06 |
| Pd-Sn/C-trisodium citrate | 6.0 | 4.76 | 212.24 | -0.702 | -0.089 | 111.51 |
| Pd-Sn/C-ammonia | 5.4 | 5.18 | 178.59 | -0.655 | -0.125 | 97.83 |
| Pd-Sn/C | 131.09 | -0.587 | -0.081 | 60.16 | ||
| Pd/C | 10.1 | 88.53 | -0.615 | -0.177 | 49.16 | |
Table 1 Physical and electrochemical patameters of Pd-Sn/C-ammonia, Pd-Sn/C-CyDTA, Pd-Sn/C-trisodium citrate and Pd/C, Pd-Sn/C*
| Catalyst | Crystallite size/nm | EASA/(m2·g-1) | Eop/V | Ep/V | ip/(mA·cm-2) | |
|---|---|---|---|---|---|---|
| XRD | TEM | |||||
| Pd-Sn/C-CyDTA | 4.5 | 5.09 | 297.62 | -0.632 | -0.046 | 122.06 |
| Pd-Sn/C-trisodium citrate | 6.0 | 4.76 | 212.24 | -0.702 | -0.089 | 111.51 |
| Pd-Sn/C-ammonia | 5.4 | 5.18 | 178.59 | -0.655 | -0.125 | 97.83 |
| Pd-Sn/C | 131.09 | -0.587 | -0.081 | 60.16 | ||
| Pd/C | 10.1 | 88.53 | -0.615 | -0.177 | 49.16 | |
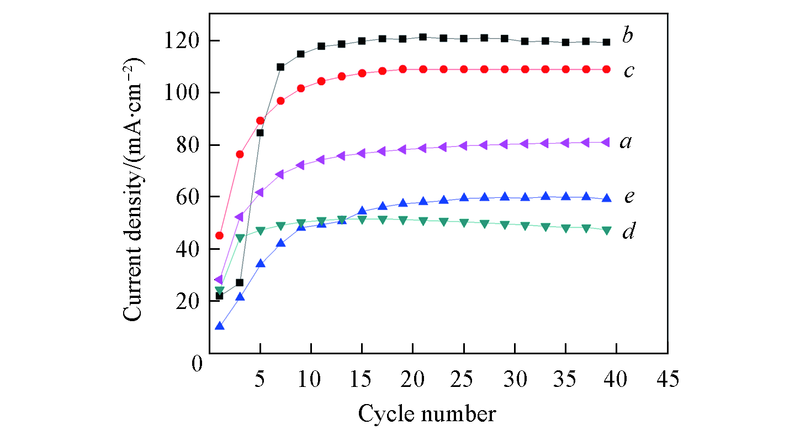
Fig.6 Cycle stabilities of Pd-Sn/C-ammonia(a), Pd-Sn/C-CyDTA(b), Pd-Sn/C-trisodium citrate(c), Pd/C(d), Pd-Sn/C(e) catalysts in 1.0 mol/L KOH+1.0 mol/L C2H5OH at a scan rate of 50 mV/s
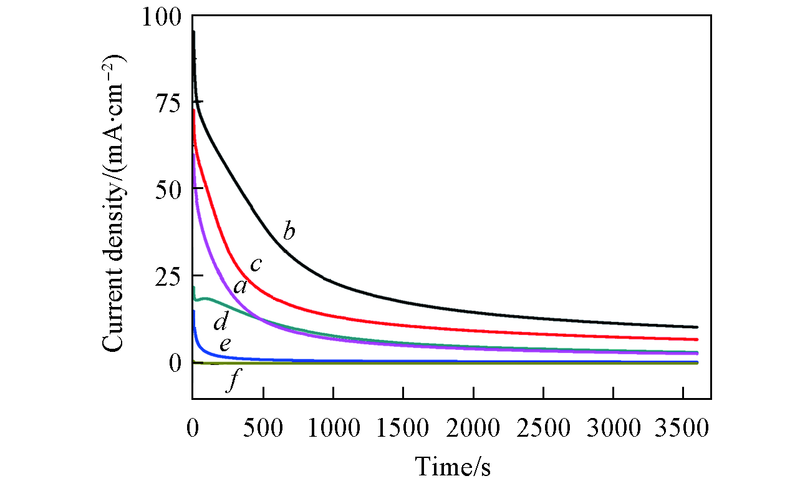
Fig.7 Chronoamperpmetric curves of Pd-Sn/C-ammonia(a), Pd-Sn/C-CyDTA(b), Pd-Sn/C-triso-dium citrate(c), Pd/C(d), Pd-Sn/C(e), Sn/C catalysts(f) in 1.0 mol/L KOH+1.0 mol/L C2H5OH solution for 3600 s at -0.25 V
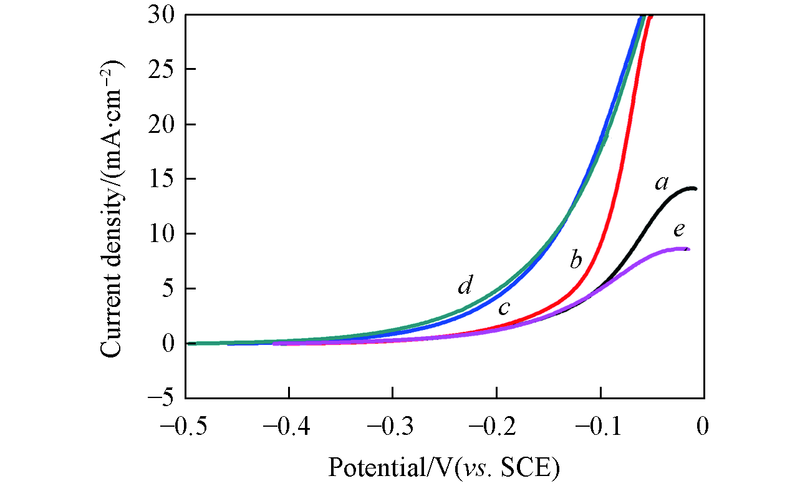
Fig.8 Polarization curves for the Pd-Sn/C-CyDTA electrocalyst in 1.0 mol/L KOH and different concentrations of ethanolc(C2H5OH)/(mol·L-1): a. 1.0; b. 2.0; c. 3.0; d. 4.0; e. 5.0.
| [1] | Guo J., Zhao T., Prabhuram J., Chen R., Wong C., Electrochimica Acta,2005, 51(4), 754—763 |
| [2] | Salgado J., Paganin V., Gonzalez E., Montemor M., Tacchini I., Ansón A., Salvador M., Ferreira P., Figueiredo F., Ferreira M., Inter. J. Hydrogen Energy,2013, 38, 901—909 |
| [3] | Xu Q. J., Zhou X. J., Li Q. X., Li J. G., Acta Physico-Chimica Sinica,2010, 26(08), 2135—2138 |
| (徐群杰, 周小金, 李巧霞, 李金光. 物理化学学报, 2010, 26(08), 2135—2138) | |
| [4] | Maiyalagan T., Scott K., J. Power Sources,2010, 195(16), 5246—5251 |
| [5] | Li Q., Xu Q., Zhou X., Li J., J. Biobased Materials and Bioenergy,2013, 7(4), 525—528 |
| [6] | Ma J., Sun H. J., Zhao H., Tang Y. W., Lu T. H., Zheng J. W., Chem. J. Chinese Universities,2011, 32(12), 2856—2860 |
| (马娟, 孙瀚君, 赵宏, 唐亚文, 陆天虹, 郑军伟. 高等学校化学学报, 2011, 32(12), 2856—2860) | |
| [7] | Zhu M., Sun G., Xin Q., Electrochimica Acta,2009, 54(5), 1511—1518 |
| [8] | Ruiz Camacho B., Morais C., Valenzuela M., Alonso-Vante N., Catalysis Today,2012, 202, 36—43 |
| [9] | Liu C. P., Yang H., Xing W., Lu T. H., Chem. J. Chinese Universities,2002, 23(7), 1367—1370 |
| (刘长鹏, 杨辉, 邢巍, 陆天虹, 高等学校化学学报, 2002, 23(7), 1367—1370) | |
| [10] | Pang H., Lu J., Chen J., Huang C., Liu B., Zhang X., Electrochimica Acta,2009, 54(9), 2610—2615 |
| [11] | Camara G., Iwasita T., J. Electroanal. Chem., 2005, 578(2), 315—321 |
| [12] | Wang H., Jusys Z., Behm R., J. Phys. Chem. B,2004, 108(50), 19413—19424 |
| [13] | Yang Y. Y., Ren J., Zhang H. X., Zhou Z. Y., Sun S. G., Cai W. B., Langmuir,2013, 29(5), 1709—1716 |
| [14] | Xu C., Liu Y., J. Power Sources,2007, 164(2), 527—531 |
| [15] | Wang Y., Shi F. F., Yang Y. Y., Cai W. B., J. Power Sources,2013, 243, 369—373 |
| [16] | Mahendiran C., Maiyalagan T., Scott K., Gedanken A., Mater. Chem. Phys., 2011, 128(3), 341—347 |
| [17] | Modibedi R. M., Masombuka T., Mathe M. K., Inter. J. Hydrogen Energy,2011, 36(8), 4664—4672 |
| [18] | Zhou W., Lee J. Y., J. Phys. Chem. C,2008, 112(10), 3789—3793 |
| [19] | Lewera A., Barczuk P. J., Skorupska K., Miecznikowski K., Salamonczyk M., Kulesza P. J., J. Electroanal. Chem., 2011, 662(1), 93—99 |
| [20] | Singh R., Singh A., Carbon,2009, 47(1), 271—278 |
| [21] | Wen Z., Yang S., Liang Y., He W., Tong H., Hao L., Zhang X., Song Q., Electrochimica Acta,2010, 56(1), 139—144 |
| [22] | Zhu F., Ma G., Bai Z., Hang R., Tang B., Zhang Z., Wang X., J. Power Sources,2013, 242, 610—620 |
| [23] | Wu B., Wang B., Deng C., Gao Y., Appl. Catal. B: Environmental,2011, 103(1), 163—168 |
| [1] | XU Xiaolong, FANG Lining, LIU Changyu, LIU Minchao, JIA Jianbo. Preparation of Z-type g-C3N4/Pt/TiO2 Nanotube Array Composite Electrode and Its Performance of Photoelectric Oxidation of Methanol [J]. Chem. J. Chinese Universities, 2021, 42(9): 2926. |
| [2] | LIN Zhouchen,HUANG Qiaoxi,LEI Ming. Fabrication and Electrocatalytic Performance of Graphene-fullerene Ammonium Iodide Composite Supported Pd Nanocatalyst for Ethanol Oxidation† [J]. Chem. J. Chinese Universities, 2019, 40(5): 1013. |
| [3] | CHEN Weimin, ZHU Zhenyu. Performances of a Hybrid Carbon Material Supported Pd Catalyst for Ethanol Electrooxidation† [J]. Chem. J. Chinese Universities, 2018, 39(2): 337. |
| [4] | CAO Xiaolu, WANG Longlong, WANG Yajun, XU Qunjie, LI Qiaoxia. Facile Preparation of Amino-modified Pd/TiO2/C Nanocatalyst and Its Electrocatalytic Performance for Ethanol Oxidation in Alkaline Solution† [J]. Chem. J. Chinese Universities, 2015, 36(6): 1187. |
| [5] | GUO Qi, LI Lin-Ru, LU Liang, JI Yun, LU Tian-Hong. Electrocatalytic Performance of Cathodic Ir-Fe/C Catalyst in Direct Methanol Fuel Cell [J]. Chem. J. Chinese Universities, 2012, 33(05): 1007. |
| [6] | LI Lin-Ru, FU Hong-Gang, LU Tian-Hong. Electrocatalytic Performance of Graphene Supported Ir Catalyst for Ammonia Oxidation [J]. Chem. J. Chinese Universities, 2012, 33(01): 102. |
| [7] | LI Huan-Zhi, SHEN Juan-Zhang, YANG Gai-Xiu, TANG Ya-Wen, LU Tian-Hong*. Anodic Pd Catalyst in Direct Formic Acid Fuel Cell and Its Electrocatalytic Stability [J]. Chem. J. Chinese Universities, 2011, 32(7): 1445. |
| [8] | XIE Guo-Fang, LU Tian-Hong, YANG Gai-Xiu, CHEN Yu, ZHOU Yi-Ming, TANG Ya-Wen*. Preparation and Mechanism of Carbon Supported Pt Catalyst Using Pt(NO3)2 and Ethylenediaminetetraacetic Acid [J]. Chem. J. Chinese Universities, 2011, 32(6): 1373. |
| [9] | ZOU Yue-Chao, LI Xia, HUANG Yong-Min*, LIU Hong-Lai. Effect of Preparation Conditions on the Catalytic Performances of PtRu/CAs Catalysts for Methanol Electro-oxidation [J]. Chem. J. Chinese Universities, 2011, 32(1): 150. |
| [10] | YANG Guo-Kai, DING Ke-Qiang*. Preparation of Pt-Pd/MWCNTs and Pd/MWCNTs Catalysts by Hydrothermal Synthesis in Room Temperature Ionic Liquids [J]. Chem. J. Chinese Universities, 2010, 31(5): 994. |
| [11] | YI Qing-Feng1*, CHEN Ai-Cheng2, ZHANG Jing-Jing1, HUANG Wu1. Electrocatalytic Activity of a Novel Nanoporous Platinum Electrode Towards Methanol Oxidation [J]. Chem. J. Chinese Universities, 2007, 28(9): 1768. |
| [12] | TANG Ya-Wen1, CAO Shuang1, CHEN Yu1, BAO Jian-Chun1, LU Tian-Hong1,2*. Effect of Structure of Carbon Nanotubes on Electrocatalytic Performance of Carbon Nanotubes Supported Pt Catalysts [J]. Chem. J. Chinese Universities, 2007, 28(5): 936. |
| [13] | WANG Qi1,2, SUN Gong-Quan1, YAN Shi-You1, WANG Guo-Xiong1, XIN Qin1, CHEN Qing-Song3, LI Jun-Tao3, JIANG Yan-Xia3, SUN Shi-Gang3. In situ Electrochemical FTIR Spectroscopy of Adsorption and Oxidation Process of Methanol on PtRu/C Electrocatalyst [J]. Chem. J. Chinese Universities, 2006, 27(11): 2123. |
| [14] | BI Zhong-He, CHENG Mo-Jie, WU He-Jin, DONG Yong-Lai, YI Bao-Lian. Performance of Intermediary Temperature Solid Oxide Fuel Cell with Methanol as Fuel [J]. Chem. J. Chinese Universities, 2005, 26(6): 1110. |
| [15] | WAN Ying, WANG Xing-Yi, LU Guan-Zhong . In Situ FTIR Study of Effect of La on CH3OH Automobile Exhaust Oxidation Mechanism on Supported Palladium Catalyst [J]. Chem. J. Chinese Universities, 2001, 22(2): 276. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||