

Chem. J. Chinese Universities ›› 2014, Vol. 35 ›› Issue (2): 377.doi: 10.7503/cjcu20130537
• Physical Chemistry • Previous Articles Next Articles
LI Haili, ZHU Hongqiao, CAO Fahe, LENG Wenhua*( )
)
Received:2013-06-06
Online:2014-02-10
Published:2013-10-23
Contact:
LENG Wenhua
E-mail:lengwh@zju.edu.cn
Supported by:CLC Number:
TrendMD:
LI Haili, ZHU Hongqiao, CAO Fahe, LENG Wenhua. Enhanced the Performance of Photoelectrochemical Oxidation of Water over BiVO4 Film Electrodes by Electrochemical Reduction Pretreatment†[J]. Chem. J. Chinese Universities, 2014, 35(2): 377.
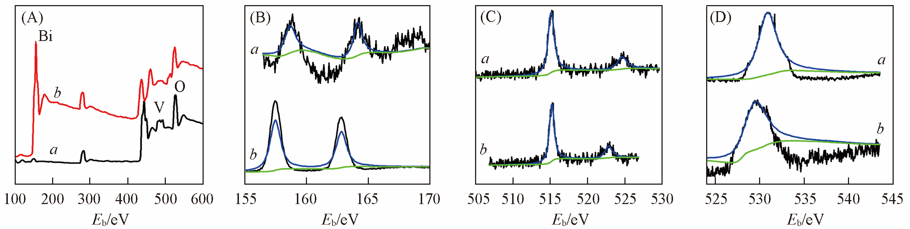
Fig.4 XPS spectra of BiVO4 electrodes without(a) and with(b) ER(A) Wide spectra; (B) Bi; (C) V; (D) O element. Dashed and dotted lines in (B)—(D) are fitted and background ones, respectively.
| Element | Eb/eV | Peak areaa | Area ratiob | Mass ratio by EDSc | ||
|---|---|---|---|---|---|---|
| without ER | with ER | without ER | with ER | |||
| B | 158.7 | 157.3 | 814 | 918 | 0.887 | 0.881 |
| B | 164.2 | 162.6 | ||||
| 516.1 | 515.2 | 892 | 965 | 0.924 | 0.9 | |
| 523.7 | 522.8 | |||||
| O1s | 520.8 | 529.4 | 3830 | 4026 | 0.951 | 0.9 |
Table 1 XPS and EDS data of BiVO4 thin films without and with ER
| Element | Eb/eV | Peak areaa | Area ratiob | Mass ratio by EDSc | ||
|---|---|---|---|---|---|---|
| without ER | with ER | without ER | with ER | |||
| B | 158.7 | 157.3 | 814 | 918 | 0.887 | 0.881 |
| B | 164.2 | 162.6 | ||||
| 516.1 | 515.2 | 892 | 965 | 0.924 | 0.9 | |
| 523.7 | 522.8 | |||||
| O1s | 520.8 | 529.4 | 3830 | 4026 | 0.951 | 0.9 |
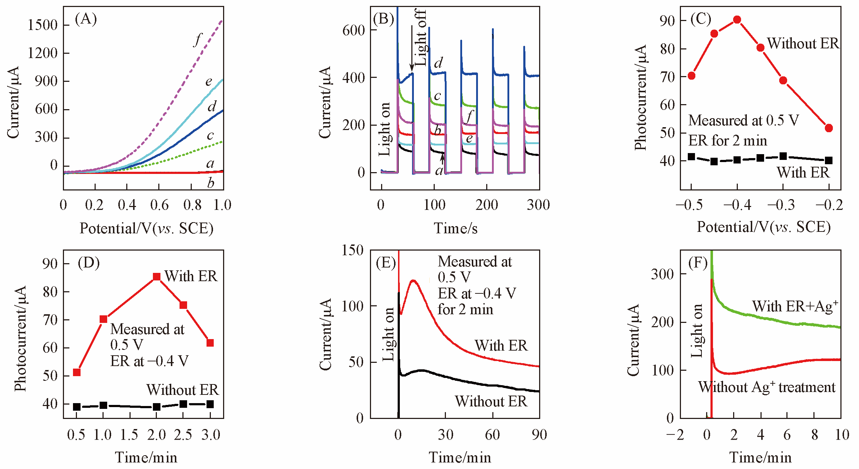
Fig.6 Photoelectrochemical performance of BiVO4 thin film electrodes without and with ER pretreatment(A) I-V curves of BiVO4 electrodes in the dark(a, b), under visible(c, d) and UV-Vis light(e, f) illumination. a, c, e. without ER(dark); b, d, f. with ER(dark); (B) corresponding I-t curves of BiVO4 electrodes to Fig.6(A). a. without ER(Vis); b. with ER(Vis); c. without ER(UV-Vis); d. with ER(UV-Vis); e. on FTO without ER(Vis); f. on FTO with ER(Vis); (C, D) photocurrent of BiVO4 electrodes versus ER potential(C) and ER time(D); (E) photoelectrochemical stability; (F) effect of immersion treatment in Ag+ containing solutions[11] on the photoelectrochemical stability of the ER treated BiVO4 electrodes. (C)—(F) with visible light illumination.
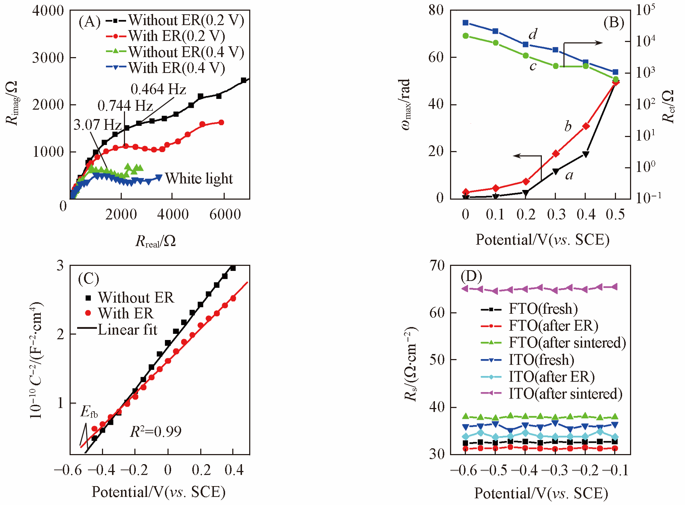
Fig.7 Typical EIS under white light(A), charge-transfer rate constant(B), Mott-Schottky curves(C) for the BiVO4 film electrodes without and with ER, and series resistance(Rs) measured at 100 kHz by EIS vs. applied potential for the fresh, ER treated(-0.5 V for 2 min) and sintered(550 ℃ for 0.5 h) ITO and FTO glass(D)(B) a, d. Without ER; b, c. with ER.(C) Efb: flatband potential, R2: relevant coefficient.
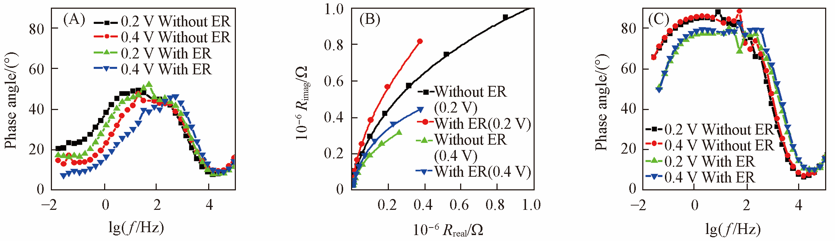
Fig.8 Typical EIS responses for the BiVO4 electrodes under white light illumination(A) and in the dark(B, C) without and with ER pretreatment(B) Nyquist; (C) bode.
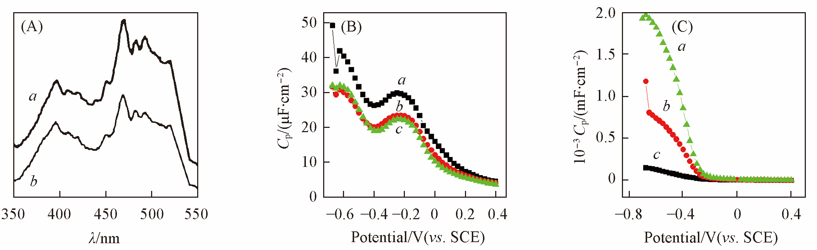
Fig.9 PL spectra of BiVO4 film electrode without(a) and with(b) ER(A) and relationship of Cp-potential in the dark for the BiVO4 thin film electrodes without(B) and with(C) ER pretreatmenta. 1 Hz; b. 5 Hz; c. 10 Hz.
| [1] | Esswein M. J., Nocera D. G., Chem. Rev., 2007, 107(10), 4022—4047 |
| [2] | Walter M. G., Warren E. L., McKone J. R., Boettcher S. W., Mi Q., Santori E. A., Lewis N. S., Chem. Rev., 2010, 110(11), 6446—6473 |
| [3] | Zou Z., Ye J., Sayama K., Arakawa H., Nature, 2001, 414(6864), 625—627 |
| [4] | Abe R., Higashi M., Domen K., J. Am. Chem. Soc., 2010, 132(34), 11828—11829 |
| [5] | Leng W. H., Barnes P. R. F., Juozapavicius M., O'Regan B. C., Durrant J. R., J. Phys. Chem. Lett., 2010, 1(6), 967—972 |
| [6] | Cowan A. J., Leng W., Barnes P. R. F., Klug D. R., Durrant J. R., Phys. Chem. Chem. Phys., 2013, 15(22), 8772—8778 |
| [7] | Fujishima A., Honda K., Nature, 1972, 238(5358), 37—38 |
| [8] | Luo W., Li Z., Yu T., Zou Z., J. Phys. Chem. C, 2012, 116(8), 5076—5081 |
| [9] | Park H. S., Kweon K. E., Ye H., Paek E., Hwang G. S., Bard A. J., J. Phys. Chem. C, 2011, 115(36), 17870—17879 |
| [10] | Cao S. W., Yin Z., Barber J., Boey F. Y. C., Loo S. C. J., Xue C., ACS Appl. Mater. Interf., 2011, 4(1), 418—423 |
| [11] | Sayama K., Nomura A., Arai T., Sugita T., Abe R., Yanagida M., Oi T., Iwasaki Y., Abe Y., Sugihara H., J. Phys. Chem. B, 2006, 110(23), 11352—11360 |
| [12] | Ye H., Park H. S., Bard A. J., J. Phys. Chem. C, 2011, 115(25), 12464—12470 |
| [13] | Lin F., Wang D., Jiang Z., Ma Y., Li J., Li R., Li C., Energy Environ. Sci., 2012, 5(4), 6400—6406 |
| [14] | Seabold J. A., Choi K. S., J. Am. Chem. Soc., 2012, 134(4), 2186—2192 |
| [15] | Abdi F. F., van de Krol R., J. Phys. Chem. C, 2012, 116(17), 9398—9404 |
| [16] | Chen J., Sun Y., Duan X. C., Zheng W. J., Chem. J. Chinese Universities, 2011, 32(3), 667—672 |
| (陈菁, 孙燕, 段小川, 郑文君.高等学校化学学报,2011, 32(3), 667—672) | |
| [17] | Hong S. J., Lee S., Jang J. S., Lee J. S., Energy Environ. Sci., 2011, 4(5), 1781—1787 |
| [18] | Long M., Cai W., Cai J., Zhou B., Chai X., Wu Y., J. Phys. Chem. B, 2006, 110(41), 20211—20216 |
| [19] | Saito R., Miseki Y., Sayama K., Chem. Commun., 2012, 48(32), 3833—3835 |
| [20] | Wetchakun N., Chaiwichain S., Inceesungvorn B., Pingmuang K., Phanichphant S., Minett A. I., Chen J., ACS Appl. Mater. Interf., 2012, 4(7), 3718—3723 |
| [21] | Kay A., Cesar I., Grätzel M., J. Am. Chem. Soc., 2006, 128(49), 15714—15721 |
| [22] | Cheng X. F., Leng W. H., Liu D. P., Xu Y. M., Zhang J. Q., Cao C. N., J. Phys. Chem. C, 2008, 112(23), 8725—8734 |
| [23] | Leng W. H., Zhang Z., Zhang J. Q., Cao C. N., J. Phys. Chem. B, 2005, 109(31), 15008—15023 |
| [24] | Leng W. H., Zhang Z., Cheng S. A., Zhang J. Q., Cao C. N., Chin. Chem. Lett., 2001, 12(11), 1019—1022 |
| [25] | Jing L., Xin B., Yuan F., Xue L., WangB., Fu H., J. Phys. Chem. B, 2006, 110(36), 17860—17865 |
| [1] | PENG Kuilin, LI Guilin, JIANG Chongyang, ZENG Shaojuan, ZHANG Xiangping. Research Progress for the Role of Electrolytes in the CO2 Electrochemical Reduction [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220238. |
| [2] | YANG Dongwei, LI Lu, WANG Qin, WANG Xiaochun, LI Qingyuan, SHI Jin. Catalytic Mechanism of Ionic Liquid for CO2 Electrochemical Reduction† [J]. Chem. J. Chinese Universities, 2016, 37(1): 94. |
| [3] | YAN Pengli, LI Ailong, ZHANG Bingqing, SHI Jingying, GAN Yang, LI Can. Electrochemical Reduction Effects on the Morphology and Electrochemical Capacity of Carbon Paper [J]. Chem. J. Chinese Universities, 2015, 36(11): 2304. |
| [4] | DING Xiao-Li, ZHANG Chao, ZHANG Yu-Zhong, CAO Zhan-Ping. Fabrication of PEBAX®2533/PSf Hollow Fiber Composition Membrane and Its Transfer Resistance [J]. Chem. J. Chinese Universities, 2012, 33(11): 2591. |
| [5] | CHEN Qing SUN Yan DUAN Xiao-Chuan ZHENG Wen-Jun*. Synthesis and Characterization of m-BiVO4 via Ionic Liquid-assisted Hydrothermal Synthesis [J]. Chem. J. Chinese Universities, 2011, 32(3): 667. |
| [6] | ZHANG Hong, REN Qi-Zhi*, HE Sheng-Yi, WANG Qing-Yu, JIANG Zong-Run. High-temperature Pyrolysis of Ketjen Black EC 300J Supported Cobalt Porphyrin Complexes as Electrocatalysis for Oxygen Reduction [J]. Chem. J. Chinese Universities, 2011, 32(2): 344. |
| [7] |
XU Yi1,2,3*, ZHANG Jian1,2, ZHANG Wen-Pin1,2,3, ZHANG Zhong-Feng1, WEN Zhi-Yu2,3 .Construction of Pretreatment Units with Esterified Silica Monolithic Column and Affinity Membrane on Micro-fluidic Chip [J]. Chem. J. Chinese Universities, 2008, 29(5): 892. |
| [8] | ZHANG Mei-Qin, LIU Hui, HU Hu, XIE Shu-Bao, JING Ping, LI Mei-Xian, GAN Liang-Bing, ZHU Zhi-Wei, KOU Yuan, SHAO Yuan-Hua*. Electrochemical Detection of C606- and C706- in Toluene at Room Temperature [J]. Chem. J. Chinese Universities, 2007, 28(4): 727. |
| [9] | CHEN Xing, CUI Da-Fu, LIU Chang-Chun, LI Hui, ZHAO Wei-Xing. Sample Pretreatment Microfluidic Chip for DNA Extraction from Rat Peripheral Blood [J]. Chem. J. Chinese Universities, 2006, 27(4): 618. |
| [10] | WANG Xing-Hua, LI Bao-Hua, XIE Fei, HUANG Feng, MA Jun, YU Ai-Min. A Novel Method of Fast Sample Pretreatment for in situ Determination of Formaldehyde in Water-soaked Foodstuffs [J]. Chem. J. Chinese Universities, 2005, 26(11): 2015. |
| [11] | WANG Jian-Hua, FANG Zhao-Lun. Lab-on-valve Mesofluidic Analytical System and Its Applications in Sample Pretreatments [J]. Chem. J. Chinese Universities, 2004, 25(S1): 135. |
| [12] | WANG Jing, FU Li-Xin, LI Jun-Hua. Effect of Pretreatment Conditions on the Activity of Catalytic Reduction of NO over Pt/Al2O3 Catalyst [J]. Chem. J. Chinese Universities, 2004, 25(6): 1090. |
| [13] | Takahashi K., Hashimoto K., Fujishima A., Omata K., Kimura N.. Electrochemical CO2 Reduction Using Gas Diffusion Electrode Containing Cu Catalyst Supported on Various Metal Oxides [J]. Chem. J. Chinese Universities, 1995, 16(S1): 189. |
| [14] | Tryk D.A., Hirota K., Hashimoto K., Fujishima A., Omata K., Kimura N.. Photoelectrochemical Reduction of Highly Concentrated CO2 Using a p-InP Electrode [J]. Chem. J. Chinese Universities, 1995, 16(S1): 218. |
| [15] | YOU Jin-Kua, ZHONG Wen-Long, LIN Zu-Geng. In situ FT-IR Spectroscopic Study of Electrochemical Reduction of Thionyl Chloride on Pt Electrode [J]. Chem. J. Chinese Universities, 1994, 15(9): 1377. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||