

Chem. J. Chinese Universities ›› 2018, Vol. 39 ›› Issue (8): 1759.doi: 10.7503/cjcu20170820
• Physical Chemistry • Previous Articles Next Articles
JIANG Tao1,*( ), WANG Ning1, PENG Shuming1, LI Mei2, HAN Wei2, CHEN Yitung3
), WANG Ning1, PENG Shuming1, LI Mei2, HAN Wei2, CHEN Yitung3
Received:2017-12-15
Online:2018-08-10
Published:2018-06-25
Contact:
JIANG Tao
E-mail:tjiang@caep.cn
Supported by:CLC Number:
TrendMD:
JIANG Tao, WANG Ning, PENG Shuming, LI Mei, HAN Wei, CHEN Yitung. Electrochemical Behaviour of Gd(Ⅲ) on Bi Electrode and Thermodynamic Data of BixGdy Intermetallic Compounds in LiCl-KCl Molten Salts†[J]. Chem. J. Chinese Universities, 2018, 39(8): 1759.
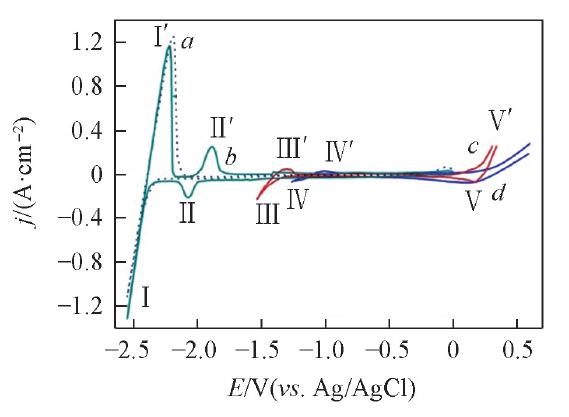
Fig.1 Cyclic voltammograms under different conditionsa. LiCl-KCl melts on W electrode; b. LiCl-KCl-GdCl3(5.2×10-5 mol/mL) melts on W electrode; c. LiCl-KCl melts on liquid Bi electrode; d. LiCl-KCl-GdCl3(5.2×10-5 mol/mL) melts on liquid Bi pool electrode. Scan rate: 0.1 V/s, SW=0.314 cm2, SBi=0.2 cm2, T=773 K.
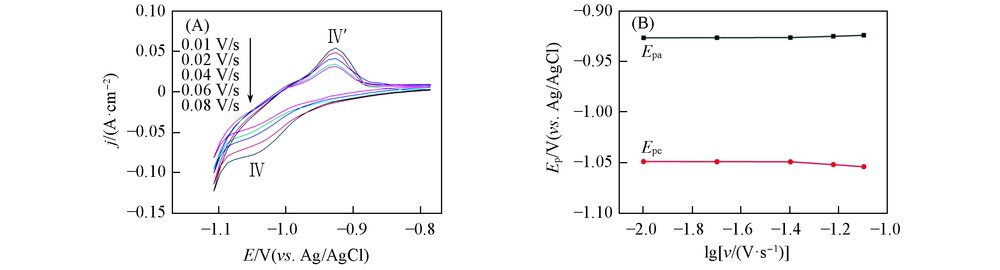
Fig.2 Cyclic voltammograms obtained in LiCl-KCl-GdCl3(5.2×10-5 mol/mL) melts on liquid Bi pool electrode at different scan rates(A) and relatioship between cathodic and anodic peak potentials with logarithm of scan rates(B)SBi=0.2 cm2, T=773 K.
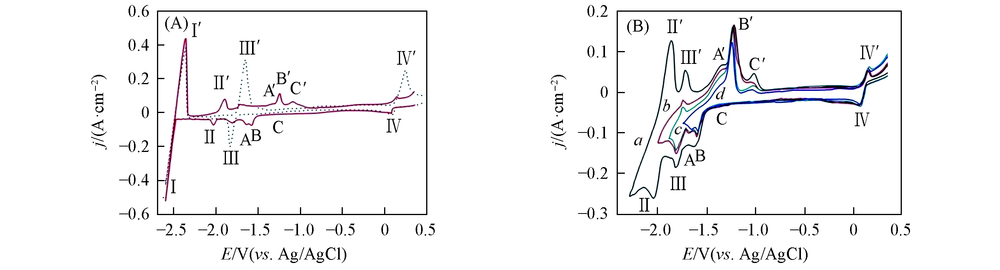
Fig.3 Cyclic voltammograms under different conditions(A) LiCl-KCl-BiCl3(3.37×10-6 mol/mL) melts on W electrode(dotted curve); LiCl-KCl-BiCl3(3.37×10-6 mol/mL)-GdCl3(8.67×10-5 mol/mL) melts on pre-deposited Bi film electrode(solid line); (B) LiCl-KCl-BiCl3(3.37×10-6 mol/mL)-GdCl3(8.67×10-5 mol/mL) melts on pre-deposited Bi film electrode at different terminal potentials. Terminal potential/V: a. -2.25; b. -2.00; c. -1.80; d. 1.75. Scan rate=0.1 V/s, S=0.314 cm2, T=773 K.
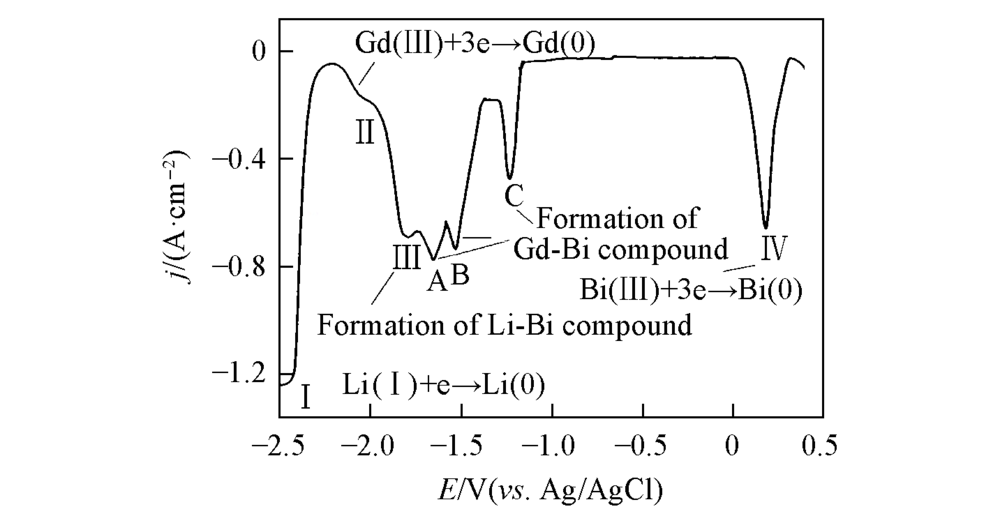
Fig.4 Square wave voltammogram obtained in LiCl-KCl-BiCl3(3.37×10-6 mol/mL)-GdCl3(8.67×10-5 mol/mL) melts on pre-deposited Bi film electrodePotential step=1 mV, frequency=20 Hz, S=0.314 cm2, T=773 K.
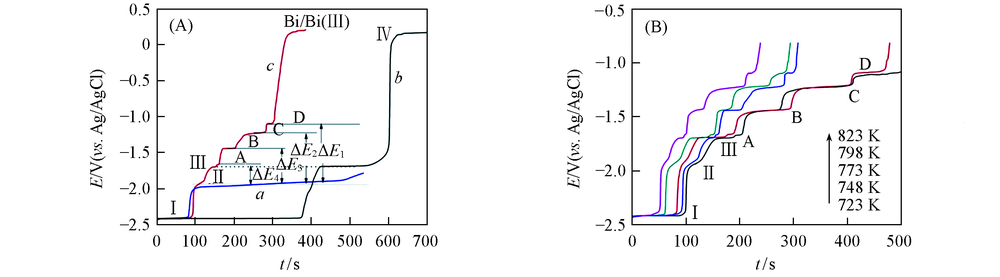
Fig.5 Open circuit chronopotentiograms of different conditions(A) a. LiCl-KCl-GdCl3(8.67×10-5 mol/mL) melts on W electrode; b. LiCl-KCl-BiCl3(3.37×10-6 mol/mL) melts on W electrode; c. LiCl-KCl-BiCl3(3.37×10-6 mol/mL)-GdCl3(8.67×10-5 mol/mL) melts on pre-deposited Bi film electrode; (B) LiCl-KCl-BiCl3(3.37×10-6 mol/mL)-GdCl3(8.67×10-5 mol/mL) melts on pre-deposited Bi film electrode at different temperatures. Deposition potential=-2.5 V, deposition time=60 s, S=0.314 cm2, T=773 K.
| Plateau* | T/K | Eeq/V(vs. Ag/AgCl) | ΔE/V[vs. Gd(Ⅲ)/Gd] | Δ | aGd |
|---|---|---|---|---|---|
| Ⅱ | 723 | -1.933±0.002 | — | — | — |
| 748 | -1.918±0.004 | — | — | — | |
| 773 | -1.903±0.002 | — | — | — | |
| 798 | -1.888±0.003 | — | — | — | |
| 823 | -1.881±0.002 | — | — | — | |
| A | 723 | -1.106±0.003 | 0.827±0.005 | -239.42±1.44 | 5.04×10-18 |
| 748 | -1.093±0.002 | 0.825±0.006 | -238.84±1.74 | 2.09×10-17 | |
| 773 | -1.081±0.002 | 0.822±0.004 | -237.97±1.16 | 8.30×10-17 | |
| 798 | -1.071±0.003 | 0.817±0.006 | -236.52±1.74 | 3.29×10-16 | |
| 823 | -1.067±0.003 | 0.814±0.005 | -235.65±1.45 | 1.10×10-15 | |
| B | 723 | -1.226±0.001 | 0.707±0.003 | -204.68±0.87 | 1.63×10-15 |
| 748 | -1.219±0.002 | 0.699±0.006 | -202.36±1.74 | 7.38×10-15 | |
| 773 | -1.209±0.003 | 0.694±0.005 | -200.91±1.45 | 2.65×10-14 | |
| 798 | -1.201±0.003 | 0.687±0.006 | -198.89±1.74 | 9.57×10-14 | |
| 823 | -1.197±0.001 | 0.684±0.003 | -198.02±0.87 | 2.70×10-13 | |
| C | 723 | -1.441±0.001 | 0.492±0.003 | -142.43±0.869 | 5.12×10-11 |
| 748 | -1.439±0.001 | 0.479±0.005 | -138.67±1.448 | 2.07×10-11 | |
| 773 | -1.438±0.002 | 0.465±0.004 | -134.62±1.158 | 8.00×10-10 | |
| 798 | -1.436±0.002 | 0.452±0.005 | -130.85±1.448 | 2.72×10-9 | |
| 823 | -1.433±0.001 | 0.448±0.003 | -129.70±0.869 | 5.86×10-9 | |
| D | 723 | -1.667±0.001 | 0.266±0.003 | -77.00±0.869 | 2.73×10-6 |
| 748 | -1.664±0.002 | 0.254±0.006 | -73.53±1.74 | 7.32×10-6 | |
| 773 | -1.662±0.003 | 0.241±0.005 | -69.77±1.45 | 1.93×10-5 | |
| 798 | -1.659±0.002 | 0.229±0.005 | -66.30±1.45 | 4.57×10-5 | |
| 823 | -1.657±0.003 | 0.224±0.005 | -64.85±1.45 | 7.66×10-5 |
Table 1 Thermodynamic properties of Gd in two-phase coexisting states at different temperatures
| Plateau* | T/K | Eeq/V(vs. Ag/AgCl) | ΔE/V[vs. Gd(Ⅲ)/Gd] | Δ | aGd |
|---|---|---|---|---|---|
| Ⅱ | 723 | -1.933±0.002 | — | — | — |
| 748 | -1.918±0.004 | — | — | — | |
| 773 | -1.903±0.002 | — | — | — | |
| 798 | -1.888±0.003 | — | — | — | |
| 823 | -1.881±0.002 | — | — | — | |
| A | 723 | -1.106±0.003 | 0.827±0.005 | -239.42±1.44 | 5.04×10-18 |
| 748 | -1.093±0.002 | 0.825±0.006 | -238.84±1.74 | 2.09×10-17 | |
| 773 | -1.081±0.002 | 0.822±0.004 | -237.97±1.16 | 8.30×10-17 | |
| 798 | -1.071±0.003 | 0.817±0.006 | -236.52±1.74 | 3.29×10-16 | |
| 823 | -1.067±0.003 | 0.814±0.005 | -235.65±1.45 | 1.10×10-15 | |
| B | 723 | -1.226±0.001 | 0.707±0.003 | -204.68±0.87 | 1.63×10-15 |
| 748 | -1.219±0.002 | 0.699±0.006 | -202.36±1.74 | 7.38×10-15 | |
| 773 | -1.209±0.003 | 0.694±0.005 | -200.91±1.45 | 2.65×10-14 | |
| 798 | -1.201±0.003 | 0.687±0.006 | -198.89±1.74 | 9.57×10-14 | |
| 823 | -1.197±0.001 | 0.684±0.003 | -198.02±0.87 | 2.70×10-13 | |
| C | 723 | -1.441±0.001 | 0.492±0.003 | -142.43±0.869 | 5.12×10-11 |
| 748 | -1.439±0.001 | 0.479±0.005 | -138.67±1.448 | 2.07×10-11 | |
| 773 | -1.438±0.002 | 0.465±0.004 | -134.62±1.158 | 8.00×10-10 | |
| 798 | -1.436±0.002 | 0.452±0.005 | -130.85±1.448 | 2.72×10-9 | |
| 823 | -1.433±0.001 | 0.448±0.003 | -129.70±0.869 | 5.86×10-9 | |
| D | 723 | -1.667±0.001 | 0.266±0.003 | -77.00±0.869 | 2.73×10-6 |
| 748 | -1.664±0.002 | 0.254±0.006 | -73.53±1.74 | 7.32×10-6 | |
| 773 | -1.662±0.003 | 0.241±0.005 | -69.77±1.45 | 1.93×10-5 | |
| 798 | -1.659±0.002 | 0.229±0.005 | -66.30±1.45 | 4.57×10-5 | |
| 823 | -1.657±0.003 | 0.224±0.005 | -64.85±1.45 | 7.66×10-5 |
| Intermetallic compound | Equation | T/K | Δ |
|---|---|---|---|
| Bi2Gd | Δ | 723 | -239.42±1.45 |
| 748 | -238.84±1.74 | ||
| 773 | -237.97±1.16 | ||
| 798 | -236.52±1.74 | ||
| 823 | -235.65±1.45 | ||
| BiGd | Δ | 723 | -222.05±1.16 |
| 748 | -220.60±1.74 | ||
| 773 | -219.44±1.30 | ||
| 798 | -217.70±1.74 | ||
| 823 | -216.84±1.16 | ||
| Bi3Gd4 | Δ | 723 | -808.57±4.34 |
| 748 | -800.47±6.66 | ||
| 773 | -792.94±5.07 | ||
| 798 | -783.97±6.66 | ||
| 823 | -780.20±4.34 | ||
| Bi3Gd5 | Δ | 723 | -885.58±5.21 |
| 748 | -874.00±8.40 | ||
| 773 | -862.71±6.51 | ||
| 798 | -850.26±8.11 | ||
| 823 | -845.05±5.79 |
Table 2 Formulas and results of calculated Gibbs free energies of formation for Bi-Gd intermetallic compounds
| Intermetallic compound | Equation | T/K | Δ |
|---|---|---|---|
| Bi2Gd | Δ | 723 | -239.42±1.45 |
| 748 | -238.84±1.74 | ||
| 773 | -237.97±1.16 | ||
| 798 | -236.52±1.74 | ||
| 823 | -235.65±1.45 | ||
| BiGd | Δ | 723 | -222.05±1.16 |
| 748 | -220.60±1.74 | ||
| 773 | -219.44±1.30 | ||
| 798 | -217.70±1.74 | ||
| 823 | -216.84±1.16 | ||
| Bi3Gd4 | Δ | 723 | -808.57±4.34 |
| 748 | -800.47±6.66 | ||
| 773 | -792.94±5.07 | ||
| 798 | -783.97±6.66 | ||
| 823 | -780.20±4.34 | ||
| Bi3Gd5 | Δ | 723 | -885.58±5.21 |
| 748 | -874.00±8.40 | ||
| 773 | -862.71±6.51 | ||
| 798 | -850.26±8.11 | ||
| 823 | -845.05±5.79 |
| Intermetallic compound | Δ (kJ·mol-1) | Δ (J·mol-1·K-1) |
|---|---|---|
| Bi2Gd | -268.10±2.53 | -39.39±3.27 |
| BiGd | -387.34±3.74 | -87.38±4.83 |
| Bi3Gd4 | -1400.22±19.96 | -395.30±25.80 |
| Bi3Gd5 | -1568.07±27.40 | -521.52±35.30 |
Table 3 Thermodynamic data of Bi-Gd intermetallic compounds in temperature range of 723—823 K
| Intermetallic compound | Δ (kJ·mol-1) | Δ (J·mol-1·K-1) |
|---|---|---|
| Bi2Gd | -268.10±2.53 | -39.39±3.27 |
| BiGd | -387.34±3.74 | -87.38±4.83 |
| Bi3Gd4 | -1400.22±19.96 | -395.30±25.80 |
| Bi3Gd5 | -1568.07±27.40 | -521.52±35.30 |
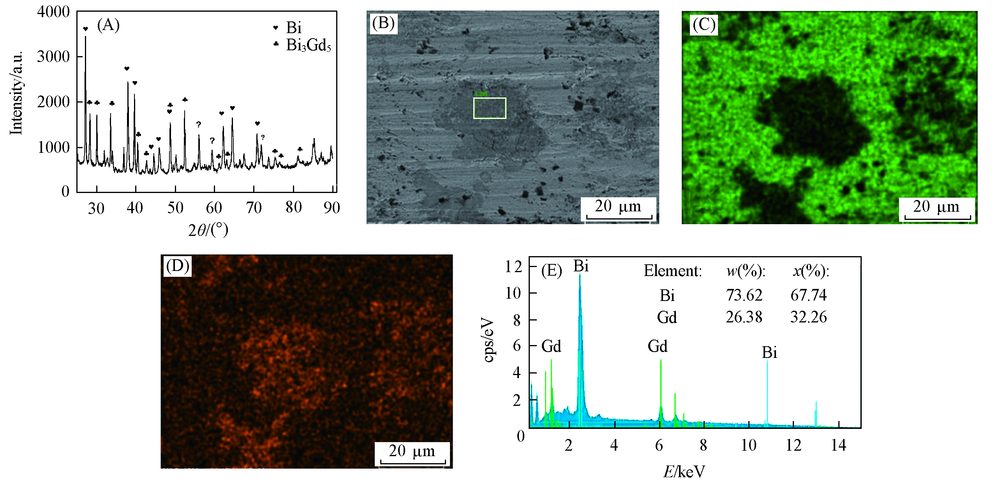
Fig.7 XRD pattern and SEM-EDS analysis of Bi-Gd alloy obtained in LiCl-KCl-GdCl3 melts by potentiostatic electrolysis(A) XRD pattern; (B) SEM image; (C) mapping analysis image of Bi; (D) mapping analysis image of Gd; (E) EDS spectrum of the framed area in (B). Electrolysis potential=-1.9 V, electrolysis time=4 h, Bi electrode, T=923 K.
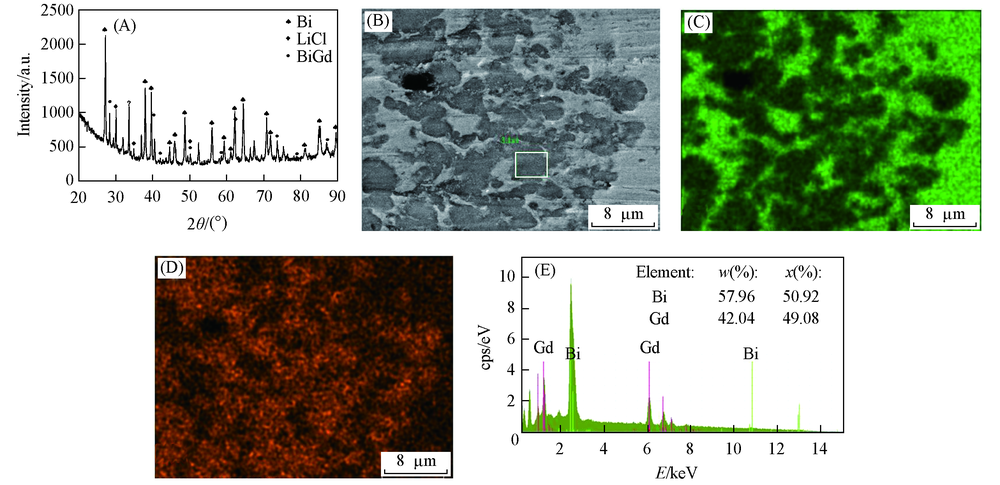
Fig.8 XRD pattern and SEM-EDS analysis of Bi-Gd alloy obtained in LiCl-KCl-GdCl3 melts by galvanostatic electrolysis(A) XRD pattern; (B) SEM image; (C) mapping analysis image of Bi; (D) mapping analysis image of Gd; (E) EDS spectrum of the framed area in (B). Current intensity=-0.5 A, electrolysis time=5.5 h, Bi electrode, T=923 K.
| [1] | Gibilaro M., Massot L., Chamelot P., Taxil P., Electrochim. Acta, 2009, 54(22), 5300—5306 |
| [2] | Kinoshita K., Koyama T., Inoue T., Ougier M., Glatz J.P., J. Phys. Chem. Solids, 2005, 66(2—4), 619—624 |
| [3] | SouČek P., Cassayre L., Malmbeck R., Mendes E., Jardin R., Glatz J. P., Radiochim. Acta, 2008, 96(4/5), 315—322 |
| [4] | Shirai O., Iwai T., Shiozawa K., Suzuki Y., Sakamura Y., Inoue T., J. Nucl. Mater., 2000, 277(2), 226—230 |
| [5] | McFarlane H. F., Lineberry M., J. Prog. Nucl. Energ., 1997, 31(1), 155—173 |
| [6] | Chen L.J., Zhang M. L., Han W., Yan Y. D., Cao P., Chem. J. Chinese Universities, 2012, 33(2), 327—330 |
| (陈丽军, 张密林, 韩伟, 颜永得, 曹鹏. 高等学校化学学报, 2012, 33(2), 327—330) | |
| [7] | Li M., Li W., Han W., Zhang M.L., Yan Y. D., Chem. J. Chinese Universities, 2014, 35(12), 2662—2667 |
| (李梅, 李炜, 韩伟, 张密林, 颜永得. 高等学校化学学报, 2014, 35(12), 2662—2667) | |
| [8] | Li M., Gu Q.Q., Han W., Zhang X. M., Sun Y., Zhang M. L., Yan Y. D, RSC Adv., 2015, 5(100), 82471—82480 |
| [9] | Castrillejo Y., Bermejo M.R., Diaz-Arocas P., Rosa F. D. L., Barrado E., Electrochemistry, 2005, 73(8), 636—643 |
| [10] | Castrillejo Y., Bermejo M.R., Arocas P. D., Martínez A. M., Barrado E., J. Electroanal. Chem., 2005, 579(2), 343—358 |
| [11] | Jiang T., Peng S.M., Li M., Pei T. T., Han W., Sun Y., Zhang M. L, Acta Phys.-Chim. Sin., 2016, 32(7), 1708—17140 |
| (姜涛, 彭述明, 李梅, 裴婷婷, 韩伟, 孙杨, 张密林. 物理化学学报, 2016, 32(7), 1708—1714) | |
| [12] | Han W., Li Z.Y., Li M., Li W. L., Zhang X. M., Yang X. G., Zhang M. L., Sun Y. J., Electrochem. Soc., 2017, 164(4), E62—E70 |
| [13] | Han W., Ji N., Wang J., Li M., Yang X., Sun Y., Zhang M., RSC Adv., 2017, 7(50), 31682—31690 |
| [14] | Vandarkuzhali S., Chandra M., Ghosh S., Samanta N., Nedumaran S., Prabhakara Reddy B., Nagarajan K., Electrochim. Acta, 2014, 145, 86—98 |
| [15] | Kato T., Inoue T., Iwai T., Arai Y., J. Nucl. Mater., 2006, 357(1), 105—114 |
| [16] | Wang L., Liu Y.L., Liu K., Tang S. L., Yuan L. Y., Lu T., Chai Z. F., Shi W. Q., J. Electrochem. Soc., 2015, 162(9), E179—E184 |
| [17] | Liu Y.L., Yuan L. Y., Kui L., Ye G. A., Zhang M. L., He H., Tang H. B., Lin R. S., Chai Z. F., Shi W. Q., Electrochim. Acta, 2014, 120, 369—378 |
| [18] | Li M., Wang J., Han W., Yang X.G., Zhang M., Sun Y., Zhang M. L., Yan Y. D., Electrochim. Acta, 2017, 228, 299—307 |
| [19] | Iizuka M., J. Electrochem. Soc., 1998, 145(1), 84—88 |
| [20] | Caravaca C., de Córdoba G., Tomás M. J., Rosado M., J. Nucl. Mater., 2007, 360(1), 25—31 |
| [21] | Tang H., Pesic B., J. Electrochem. Soc., 2014, 161(9), D429—D436 |
| [22] | Bermejo M.R., Gómez J., Medina J., Martínez A. M., Castrillejo Y., J. Electroanal. Chem., 2006, 588(2), 253—266 |
| [23] | Nourry C., Massot L., Chamelot P., Taxil P., Electrochim. Acta, 2008, 53(5), 2650—2655 |
| [24] | Nourry C., Massot L., Chamelot P., Taxil P., J. Appl. Electrochem., 2009, 39(12), 2359—2367 |
| [25] | Nourry C., Massot L., Chamelot P., Taxil P., J. Appl. Electrochem., 2009, 39(6), 927—933 |
| [26] | Zhou W., Liu Y.L., Liu K., Liu Z. R., Yuan L. Y., Wang L., Feng Y. X., Chai Z. F., Shi W. Q., J. Electrochem. Soc., 2015, 162(10), D531—D539 |
| [27] | Seon F., Picard G., Tremillon B., Electrochim. Acta, 1983, 28(2), 209—215 |
| [28] | Shibata H., Hayashi H., Akabori M., Arai Y., Kurata M., J. Phys. Chem. Solids, 2014, 75(8), 972—976 |
| [29] | Castrillejo Y., Vega A., Vega M., Hernández P., Rodriguez J.A., Barrado E., Electrochim. Acta, 2014, 118, 58—66 |
| [30] | Wang J.S., Li C. R., Guo C. P., Du Z. M., Wu B, Calphad., 2013, 41, 1—5 |
| [1] | LIU Mei, YAN Wei-Wei, ZANG Na, RUAN Wen-Juan*, ZHU Zhi-Ang. Synthesis and Properties of Novel Porphyrin Modified by Pyridine Derivative [J]. Chem. J. Chinese Universities, 2009, 30(8): 1501. |
| [2] | XU Xiu-Ming, LI Chang-Zhi, ZHAO Zong-Bao, WANG Jun-De, LI Hai-Yang*. Molecular Interactions Between Imidazole-based Ionic Liquids and Gases Using Quartz Crystal Microbalance [J]. Chem. J. Chinese Universities, 2009, 30(7): 1322. |
| [3] | GAO Hong-Xu1, ZHAO Feng-Qi1*, HU Rong-Zu1, XU Kang-Zhen2, ZHANG Hai3, WANG Peng4, DU Zhi-Ming4, XU Si-Yu1, YI Jian-Hua1, MA Hai-Xia2, CHANG Chun-Ran2, SONG Ji-Rong2,5. Specific Heat Capacity, Thermodynamic Properties, Adiabatic Time-to-Explosion and Thermal Sensitivity Probability Density Distribution of 3,4-dinitrofurazanfuroxan(DNTF) [J]. Chem. J. Chinese Universities, 2008, 29(5): 981. |
| [4] | XIA Qi-Ying, XIAO He-Ming, JU Xue-Hai, GONG Xue-Dong . Theoretical Study on the Structures and Properties of Group ⅢA Metallic Azide Clusters [J]. Chem. J. Chinese Universities, 2005, 26(5): 922. |
| [5] | SUN Qiao-Yu, ZHANG Xiao-Gang, LI Xiao-Hong, LI Hu-Lin . Solvent Effect on the Electrochemical Behavior of Self-assembled Monolayers [J]. Chem. J. Chinese Universities, 2001, 22(10): 1693. |
| [6] | DAI Zhang-wen, WANG Zhen-Min, XU Yan, DAI Xiao-Hong. Estimation of the Standard Formation Enthalpy of Carbonates [J]. Chem. J. Chinese Universities, 1996, 17(12): 1937. |
| [7] | DAI Zhang-Wen, DAI Xiao-Hong. Studies on the Correlation of Bond Effect and Thermodynamic Property of Carbonates [J]. Chem. J. Chinese Universities, 1994, 15(8): 1210. |
| [8] | Hao Ce, Shi Cai-yun, Chen Zong-qi, Xiao Gui-shi, Song Jiu-wei, Cheng De-shu. Thermodynamic Properties of Microemulsion Composed of Nonionic Surfactants(Ⅱ)--The Effect of Alkyl Chain Length of Alcohol and Oxyethylene Unit of Nonionic Surfactants [J]. Chem. J. Chinese Universities, 1991, 12(10): 1357. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||