

Chem. J. Chinese Universities ›› 2018, Vol. 39 ›› Issue (3): 435.doi: 10.7503/cjcu20170507
• Analytical Chemistry • Previous Articles Next Articles
WANG Yi1, ZHANG Ping1, WU Shengxiu1, SUN Yuanyuan1, ZHAO Tong1, LIU Shixi2,*( )
)
Received:2017-07-26
Online:2018-03-10
Published:2018-01-17
Contact:
LIU Shixi
E-mail:shxliu@ynu.edu.cn
Supported by:CLC Number:
TrendMD:
WANG Yi, ZHANG Ping, WU Shengxiu, SUN Yuanyuan, ZHAO Tong, LIU Shixi. Fragmentation Mechanism of Two Kinds of Violated Even-electron Rule Compounds with Doubly Charged Ions in Mass Spectrometry†[J]. Chem. J. Chinese Universities, 2018, 39(3): 435.
| Compound | R1 | X1 | X2 | X3 | X4 | R2 |
|---|---|---|---|---|---|---|
| 1 | C3H7 | F | H | H | H | C2H5 |
| 2 | C3H7 | H | H | H | H | C4H9 |
| 3 | C3H7 | F | H | H | H | C3H7 |
| 4 | C3H7 | F | H | H | H | C4H9 |
| 5 | C3H7 | F | H | H | H | C5H11 |
| 6 | C3H7 | H | H | F | F | C2H5 |
| 7 | C2H5 | F | F | H | H | C3H7 |
| 8 | C3H7 | H | H | F | F | C4H9 |
| 9 | C4H9 | F | F | H | H | C3H7 |
Table 1 Structures and stereochemistry of alkyl biphenyl compounds with C≡≡C central-bridge-bond*
| Compound | R1 | X1 | X2 | X3 | X4 | R2 |
|---|---|---|---|---|---|---|
| 1 | C3H7 | F | H | H | H | C2H5 |
| 2 | C3H7 | H | H | H | H | C4H9 |
| 3 | C3H7 | F | H | H | H | C3H7 |
| 4 | C3H7 | F | H | H | H | C4H9 |
| 5 | C3H7 | F | H | H | H | C5H11 |
| 6 | C3H7 | H | H | F | F | C2H5 |
| 7 | C2H5 | F | F | H | H | C3H7 |
| 8 | C3H7 | H | H | F | F | C4H9 |
| 9 | C4H9 | F | F | H | H | C3H7 |
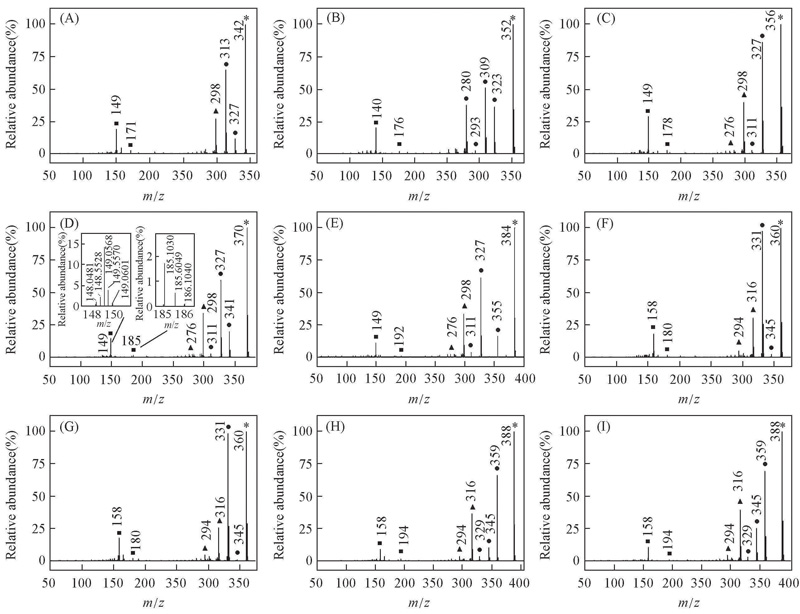
Fig.1 EI mass spectra of alkyl biphenyl compounds with C≡≡C central-bridge-bond for compounds 1—9(A—I) ■ Doubly charged ion; ▲ even electron ion; ● odd electron ion; * molecular ion. Insets of (D) arethe expanded EI mass spectra, showing the presence of 13C isotope for doubly charged ion.
| Type of ion | Theoretical value | Measured value | 103 Error | Elemental composition |
|---|---|---|---|---|
| [odd] +· | 370.2091 | 370.2060 | 3.1 | C27H27F+· |
| [even] + | 341.1700 | 341.1736 | 3.6 | C25H22F+ |
| [even] + | 327.1544 | 327.1513 | 3.1 | C24H20F+ |
| [even] + | 311.1231 | 311.1198 | 3.3 | C23H16F+ |
| [odd] +· | 298.1152 | 298.1147 | 0.5 | C22H15F+· |
| [odd] +· | 276.0934 | 276.0896 | 3.8 | C22 |
| [even] 2+ | 185.1043 | 185.1030 | 1.3 | C27H27F2+ |
| [even] 2+ | 149.0573 | 149.0568 | 0.5 | C22H15F2+ |
Table 2 Results of Q-TOF-HRMS of compound 4
| Type of ion | Theoretical value | Measured value | 103 Error | Elemental composition |
|---|---|---|---|---|
| [odd] +· | 370.2091 | 370.2060 | 3.1 | C27H27F+· |
| [even] + | 341.1700 | 341.1736 | 3.6 | C25H22F+ |
| [even] + | 327.1544 | 327.1513 | 3.1 | C24H20F+ |
| [even] + | 311.1231 | 311.1198 | 3.3 | C23H16F+ |
| [odd] +· | 298.1152 | 298.1147 | 0.5 | C22H15F+· |
| [odd] +· | 276.0934 | 276.0896 | 3.8 | C22 |
| [even] 2+ | 185.1043 | 185.1030 | 1.3 | C27H27F2+ |
| [even] 2+ | 149.0573 | 149.0568 | 0.5 | C22H15F2+ |
| Compd. | R1 | X1 | X2 | X3 | X4 | X5 | Compd. | R1 | X1 | X2 | X3 | X4 | X5 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1a | C2H5 | H | F | OCF2CFCF2 | F | H | 1b | CH3 | H | F | F | F | H |
| 2a | C3H5 | H | F | F | F | H | 2b | C2H5 | H | F | F | F | H |
| 3a | CH3 | H | H | OCF3 | H | H | 1c | H | H | F | F | F | H |
| 4a | C2H5 | H | H | OCF3 | H | H | 2c | C3H7 | H | F | F | F | H |
| 5a | C4H9 | H | F | F | F | H | 3c | C4H9 | H | F | F | F | H |
| 6a | C3H7 | H | F | F | F | H | 4c | C5H11 | H | F | F | F | H |
| 7a | C2H5 | H | F | F | F | H | 5c | C6H13 | H | F | F | F | H |
| 8a | C2H5 | CH3 | F | F | F | H | 6c | C5H11 | H | F | F | F | H |
| 9a | No Replace | H | F | F | F | H |
Table 3 Structures and stereochemistry of alkyl biphenyl compounds with CF2O central-bridge-bond*
| Compd. | R1 | X1 | X2 | X3 | X4 | X5 | Compd. | R1 | X1 | X2 | X3 | X4 | X5 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1a | C2H5 | H | F | OCF2CFCF2 | F | H | 1b | CH3 | H | F | F | F | H |
| 2a | C3H5 | H | F | F | F | H | 2b | C2H5 | H | F | F | F | H |
| 3a | CH3 | H | H | OCF3 | H | H | 1c | H | H | F | F | F | H |
| 4a | C2H5 | H | H | OCF3 | H | H | 2c | C3H7 | H | F | F | F | H |
| 5a | C4H9 | H | F | F | F | H | 3c | C4H9 | H | F | F | F | H |
| 6a | C3H7 | H | F | F | F | H | 4c | C5H11 | H | F | F | F | H |
| 7a | C2H5 | H | F | F | F | H | 5c | C6H13 | H | F | F | F | H |
| 8a | C2H5 | CH3 | F | F | F | H | 6c | C5H11 | H | F | F | F | H |
| 9a | No Replace | H | F | F | F | H |
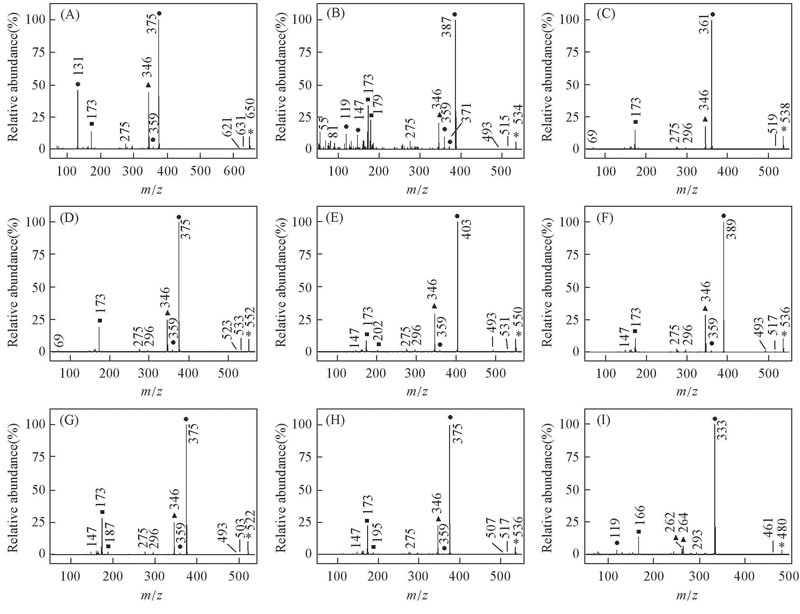
Fig.2 EI mass spectra of alkyl biphenyl compounds [a] with CF2O central-bridge-bond for compounds 1a—9a(A—I) ■ Doubly charged ion; ▲ even electron ion; ● odd electron ion; * molecular ion.
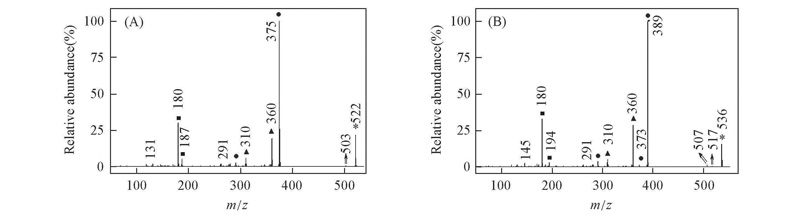
Fig.3 EI mass spectra of alkyl biphenyl compounds [b] with CF2O central-bridge-bond for compounds 1b(A) and 2b(B) ■ Doubly charged ion; ▲ even electron ion; ● odd electron ion; * molecular ion.
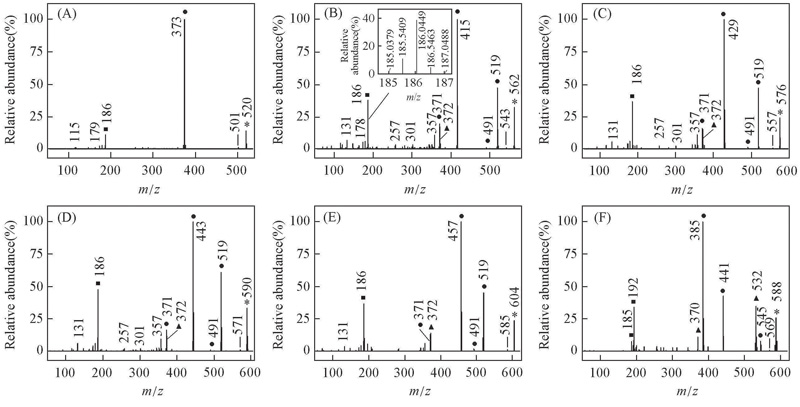
Fig.4 EI mass spectra of alkyl biphenyl compounds [c] with CF2O central-bridge-bond for compounds 1c—6c(A—F) ■ Doubly charged ion; ▲ even electron ion; ● odd electron ion; * molecular ion. Insets of (B) is the expanded EI mass spectrum, showing the presence of 13C isotope for doubly charged ion.
| Type of ion | Theoretical value | Measured value | 103 Error | Elemental composition |
|---|---|---|---|---|
| [odd]+· | 562.1537 | 562.1592 | 5.5 | C31H22F8O+· |
| [even]+ | 519.0990 | 519.0972 | 1.8 | C28H15F8O+ |
| [even]+ | 415.1480 | 415.1496 | 1.6 | C25H20 |
| [odd]+· | 372.0932 | 372.0901 | 3.1 | C22H13 |
| [even]+ | 371.0854 | 371.0894 | 4.0 | C22H12 |
| [even] 2+ | 186.0463 | 186.0449 | 1.4 | C22H13 |
Table 4 Results of Q-TOF-HRMS of compound 2c
| Type of ion | Theoretical value | Measured value | 103 Error | Elemental composition |
|---|---|---|---|---|
| [odd]+· | 562.1537 | 562.1592 | 5.5 | C31H22F8O+· |
| [even]+ | 519.0990 | 519.0972 | 1.8 | C28H15F8O+ |
| [even]+ | 415.1480 | 415.1496 | 1.6 | C25H20 |
| [odd]+· | 372.0932 | 372.0901 | 3.1 | C22H13 |
| [even]+ | 371.0854 | 371.0894 | 4.0 | C22H12 |
| [even] 2+ | 186.0463 | 186.0449 | 1.4 | C22H13 |
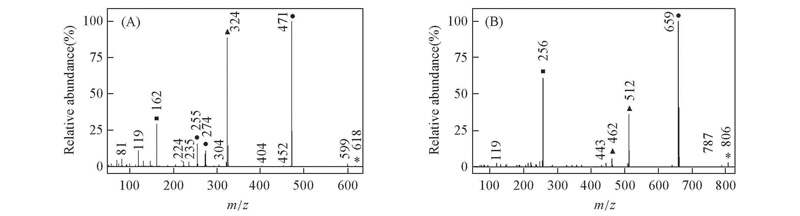
Fig.5 EI mass spectra of biphenyl compounds with double CF2O central-bridge-bond for compounds 1d(A) and 2d(B) ■ Doubly charged ion; ▲ even electron ion; ● odd electron ion; * molecular ion.
| [1] | Mathur B. P., Abbey L. E., Burgess E. M., Moran T. F., Org. Mass Spectrom., 1980, 15(6), 312—316 |
| [2] | Mathur B. P., Burgess E. M., Bostwick D. E., Moran T. F., Org. Mass Spectrom., 1981, 16(1), 92—96 |
| [3] | Jones B. E., Abbey L. E., Chatham H. L., Hanner A. W., Teleshfsky L. A., Burgess E. M., Moran T. F., Org. Mass Spectrom., 1982, 17(1), 10—18 |
| [4] | Hanner A. W., Abbey L. E., Bostwick D. E., Burgess E. M., Moran T. F., Org. Mass Spectrom., 1982, 17(1), 19—28 |
| [5] | Teleshefsky L. A., Jones B. E., Abbey L. E., Bostwick D. E., Burgess E. M., Moran T. F., Org. Mass Spectrom., 1982, 17(106), 481—492 |
| [6] | Appling J. R., Jones B. E., Abbey L. E., Bostwick D. E., Moran T. F., Org. Mass Spectrom., 1983, 18(7), 282—294 |
| [7] | Appling J. R., Musier K. M., Moran T. F., Org. Mass Spectrom., 1984, 19(9), 412—422 |
| [8] | Appling J. R., Burdick G. W., Moran T. F., Org. Mass Spectrom., 1985, 20(5), 343—350 |
| [9] | Shamma M., Dudock B. S., Cava M. P., Rao K. V., Dalton D. R., Dejongh D. C., Shrader S. R., Chem. Commun., 1966, 1(1), 7—8 |
| [10] | McLafferty F., Tureek F., Interpretation of Mass Spectra, University Science Books, California, 1993, 115—116 |
| [11] | Gross J.H., Mass Spectrometry, Science Press, Beijing, 2012, 257—259 |
| [12] | Karni M., Mandelbaum A., Org. Mass Spectrom., 1980, 15(2), 53—64 |
| [13] | Bowen R. D., Harrison A. G., Org. Mass Spectrom., 1981, 16(16), 180—182 |
| [14] | Ceraulo L., Agozzino P., Ferrugia M., Lamartina L., Natoli M. C., Org. Mass Spectrom., 1991, 26(4), 279—286 |
| [15] | Attygalle A. B., Bialecki J. B., Nishshanka U., Weisbecker C. S., Ruzicka J., J. Mass Spectrom., 2008, 43(9), 1224—1234 |
| [16] | Vessecchi R., Carollo C. A., Lopes J. N. C., Crotti A. E. M., Lopes N. P., Galembeck S. E., J. Mass Spectrom., 2009, 44(8), 1224—1233 |
| [17] | Cai Y., Mo Z., Rannulu N. S., Guan B., Kannupal S., Gibb B. C., Cole R. B., J. Mass Spectrom., 2010, 45(3), 235—240 |
| [18] | Chai Y. F., Gan S. F., Pan Y. J., Acta Chim. Sinica, 2012, 70(17), 1805—1811 |
| (柴云峰, 甘世凤, 潘远江.化学学报,2012, 70(17), 1805—1811) | |
| [19] | Nizigiyimana L., Rajan P. K., Haemers A., Claeys M., Derrick P. J., Rapid Commun. Mass Spectrom., 1997, 11(16), 1808—1812 |
| [20] | Ji H.Y., Synthesis and Properties of Novel Polyaryls, East China Normal University, Shanghai, 2009 |
| (冀海英. 新型联芳共轭有机化合物的合成及其性能研究, 上海: 华东师范大学, 2009) | |
| [21] | Gao A. A., Zheng Y. Y., Du W. S., Chinese Journal of Liquid Crystals and Displays, 2014, 29(2), 159—171 |
| (高嫒嫒, 郑远洋, 杜渭松.液晶与显示,2014, 29(2), 159—171) | |
| [22] | Meng F. B., Lian J., Gao Y. M., Progress in Chemistry, 2008, 20(4), 499—507 |
| (孟凡宝, 廉娇, 高永梅.化学进展,2008, 20(4), 499—507) | |
| [23] | Gao H.J., Liquid Crystal Chemistry, Tsinghua University Press, Beijing, 2011 |
| (高鸿锦. 液晶化学, 北京:清华大学出版社, 2011) | |
| [24] | Liu Y., Zhang Z. Y., Ren Z. D., Chinese Journal of Liquid Crystals and Displays, 2010, 25(4), 490—493 |
| (刘运, 张智勇, 任占冬.液晶与显示,2010, 25(4), 490—493) | |
| [25] | Zhang F. M., Han Y. H., Shang H. Y., Chinese Journal of Liquid Crystals and Displays, 2010, 25(4), 510—514 |
| (张芳苗, 韩耀华, 尚洪勇.液晶与显示,2010, 25(4), 510—514) | |
| [26] | Wang Y., Li M., Journal of Chinese Mass Spectrometry Society, 2015, 36(3), 255—260 |
| (王毅, 李敏.质谱学报,2015, 36(3), 255—260) | |
| [27] | Wang Y., Zhang P., Wu S. X., Chinese Journal of Liquid Crystals and Displays, 2016, 31(11), 1046—1054 |
| (王毅, 张苹, 吴生秀.液晶与显示,2016, 31(11), 1046—1054) | |
| [28] | Wang C.H., Techniques and Methods in Organic Mass Spectrometry, China Light Industry Press, Beijing, 2011 |
| (王聪慧. 有机质谱技术与方法, 北京: 中国轻工业出版社, 2011) | |
| [29] | Alex A., Harvey S., Parsons T., Pullen F. S., Wright P., Riley J. A., Rapid Commun. Mass Spectrom., 2009, 23(17), 2619—2627 |
| [1] | DING Jian-Hua, WANG Xing-Xiang, ZHANG Hui, PAN Su-Su, LUO Ming-Biao, LI Jian-Qiang, CHEN Huan-Wen*. Extrative Electrospray Ionization Tandem Mass Spectrometry of Apigenin [J]. Chem. J. Chinese Universities, 2011, 32(8): 1714. |
| [2] | LUO Xiao, OUYANG Yong-Zhong, LIANG Yi-Zeng*, WANG Qin. Interpretation of Mass Spectral Characteristic Fragmentation Mechanisms of Indole Alkaloids Through Determining the Initial Ionization Site [J]. Chem. J. Chinese Universities, 2010, 31(8): 1522. |
| [3] | . Application of Accurate Mass and Elemental Composition Determination for Pesticides Identification Using a Unit Mass Resolution Gas Chromatography/Mass Spectrometry [J]. Chem. J. Chinese Universities, 2010, 31(12): 2383. |
| [4] | YU A-Juan, WEI Kun, WU Yang-Jie*. Studies on Cyclopalladated Ferrocenylimine-phosphine Complexes by Electrospray Ionization Trap Mass Spectrometry [J]. Chem. J. Chinese Universities, 2007, 28(5): 881. |
| [5] | DONG Hong-Juan1,2, LIU Zhi-Qiang1, SONG Feng-Rui1, YU Zhan1, LI Hui-Lin1, LIU Shu-Ying1. Studies on Paeoniflorin by Electrospray Ionization Tandem Mass Spectrometry [J]. Chem. J. Chinese Universities, 2006, 27(11): 2066. |
| [6] | Zeper Abliz, Takavama Mitsuo, Ueda Toyotoshi. Structural Analysis of Condensed Polycyclic Aromatic Compounds by Tandem Mass Spectrometry [J]. Chem. J. Chinese Universities, 1996, 17(9): 1356. |
| [7] | ZHAN Dong-Liang, ZHU Yu-Fen, LIU Shu-Ying, XIN Bao-Min, JIA Wei-Ping, ZHANG Fa-Yi, HUANG Cheng-Yi, SUN Hong-Wei. Fragmentations of Some Doubly Charged Ions in the Gas Phase [J]. Chem. J. Chinese Universities, 1993, 14(9): 1270. |
| [8] | LI Zhi-li, LIU Shu-ying, PENG Jia-rou, ZHANG Hai-ying. A Study of Ion Kinetic Energy Spectrometry of Doubly Charged Ions and Singly Charged Ions of o-,m-,p-DimethylBenzenes in Gas Phase [J]. Chem. J. Chinese Universities, 1992, 13(7): 960. |
| [9] | Yang Yinghua, Jiang Yunfei, Zhao Bing, Liu Juzheng . Studies on Mass Spectrometry of Methylated Pentadiene [J]. Chem. J. Chinese Universities, 1988, 9(7): 716. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||