

Chem. J. Chinese Universities ›› 2014, Vol. 35 ›› Issue (12): 2567.doi: 10.7503/cjcu20140395
• Organic Chemistry • Previous Articles Next Articles
ZANG Hao1, SUN Jiaming1, HUANG Xiaoguang2, JI Yang2, DAI Tingting1, GAO Xiaochen1, LI Xiaodong2,*( ), ZHANG Hui1,*(
), ZHANG Hui1,*( )
)
Received:2014-04-25
Online:2014-12-10
Published:2014-11-29
Contact:
LI Xiaodong,ZHANG Hui
E-mail:baoer88@126.com;zhanghui_8080@163.com
Supported by:CLC Number:
TrendMD:
ZANG Hao, SUN Jiaming, HUANG Xiaoguang, JI Yang, DAI Tingting, GAO Xiaochen, LI Xiaodong, ZHANG Hui. Synthesis and Biological Activity of N-substituted Carnosine Amide Derivatives†[J]. Chem. J. Chinese Universities, 2014, 35(12): 2567.
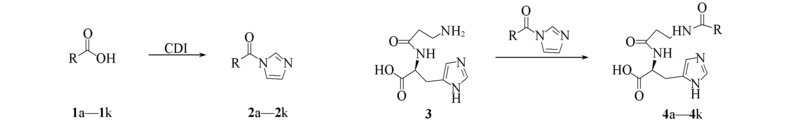
Scheme 1 Synthetic routes of the carnosine derivatives a. R=—C3H7; b. R=—C6H13; c. R=—C8H17; d. R=—C6H5; e. R=—C6H4—CH3; f. R=—C6H4—C(CH3)3; g. R=—C6H4—Cl; h. R=—C6H4—C6H5; i. R=—CH2—C6H5; j. R=—CH2CH2—C6H5; k. R= CDI: N,N'-carbonyldiimidazole.
| Compd. | Appearance | Yield(%) | m.p./℃ | UV-Vis, λmax/nm | HR-MS([M+H]+), m/z |
|---|---|---|---|---|---|
| 4a | White solid | 70.2 | 188—190 | 201 | 297.1503 |
| 4c | White solid | 58.5 | 219—220 | 213 | 367.2246 |
| 4e | White solid | 73.4 | 258—259 | 205, 239 | 345.1475 |
| 4f | White solid | 94.2 | 156—158 | 205, 239 | 387.1927 |
| 4g | White solid | 86.6 | 264—265 | 206, 238 | 365.0960 |
| 4h | White solid | 52.3 | 263—265 | 206, 269 | 407.1637 |
| 4i | White solid | 55.7 | 239—241 | 215, 258 | 345.1479 |
| 4j | White solid | 56.9 | 243—245 | 216, 258 | 359.1643 |
| 4k | White solid | 81.2 | 190—191 | 207 | 415.1373 |
Table 1 Appearance, yields, melting points, UV-Vis and HR-MS for compounds 4a, 4c, 4e—4k
| Compd. | Appearance | Yield(%) | m.p./℃ | UV-Vis, λmax/nm | HR-MS([M+H]+), m/z |
|---|---|---|---|---|---|
| 4a | White solid | 70.2 | 188—190 | 201 | 297.1503 |
| 4c | White solid | 58.5 | 219—220 | 213 | 367.2246 |
| 4e | White solid | 73.4 | 258—259 | 205, 239 | 345.1475 |
| 4f | White solid | 94.2 | 156—158 | 205, 239 | 387.1927 |
| 4g | White solid | 86.6 | 264—265 | 206, 238 | 365.0960 |
| 4h | White solid | 52.3 | 263—265 | 206, 269 | 407.1637 |
| 4i | White solid | 55.7 | 239—241 | 215, 258 | 345.1479 |
| 4j | White solid | 56.9 | 243—245 | 216, 258 | 359.1643 |
| 4k | White solid | 81.2 | 190—191 | 207 | 415.1373 |
| Compd. | 1H NMR(D2O, 600 MHz), δ | 13C NMR(D2O, 150 MHz), δ |
|---|---|---|
| 4a | 8.45(s, 1H), 7.16(s, 1H), 4.41(dd, J=7.8, 4.8 Hz, 1H), 3.32(q, J=6.6 Hz, 2H), 3.16(q, J=4.8 Hz, 1H), 3.00(q, J=8.4 Hz, 1H), 2.40(t, J=6.6 Hz, 2H), 2.09(t, J=7.2 Hz, 2H), 1.47(q, J=7.2 Hz, 2H), 0.78(t, J=7.2 Hz, 3H) | 176.3, 175.6, 172.5, 132.5, 129.2, 115.9, 53.3, 36.8, 34.9, 34.3, 26.7, 18.1, 11.8 |
| 4c | 8.47(s, 1H), 7.17(s, 1H), 4.42(t, J=7.8, 5.4 Hz, 1H), 3.33(q, J=6.6 Hz, 2H), 3.17(q, J=5.4 Hz, 1H), 3.01(q, J=7.8 Hz, 1H), 2.41(t, J=6.6 Hz, 2H), 2.12(t, J=7.2 Hz, 2H), 1.47(s, 2H), 1.19(s, 10H), 0.78(t, J=7.2 Hz, 3H) | 176.5, 175.5, 172.4, 132.5, 129.2, 115.9, 53.3, 34.9, 34.9, 34.3, 30.2, 27.5, 27.4, 27.3, 26.7, 24.5, 21.2, 12.6 |
| 4e | 8.17(s, 1H), 7.52(d, J=7.8 Hz, 2H), 7.28(d, J=8.4 Hz, 2H), 7.05(s, 1H), 4.44(s, 1H), 3.54(q, J=6.0 Hz, 2H), 3.13(t, J=4.2 Hz, 1H), 2.98(q, J=8.4 Hz, 1H), 2.56(d, J=6.6 Hz, 1H), 2.50(d, J=6.6 Hz, 1H), 2.34(s, 1H) | 175.6, 172.7, 169.7, 142.5, 132.2, 129.6, 129.1, 128.6, 128.6, 126.2, 126.2, 115.5, 53.0, 35.5, 34.5, 26.5, 19.7 |
| 4f | 8.15(s, 1H), 7.59(d, J=8.4 Hz, 2H), 7.53(d, J=8.4 Hz, 2H), 7.06(s, 1H), 4.45(dd, J=8.4, 4.8 Hz, 1H), 3.52—3.58(m, 2H), 3.14(q, J=4.8 Hz, 1H), 2.97(q, J=8.4 Hz, 1H), 2.51—2.57(m, 2H), 1.26(s, 9H) | 175.6, 172.7, 169.5, 155.5, 132.1, 129.6, 129.1, 126.1, 126.1, 125.0, 125.0, 115.6, 53.0, 35.5, 34.5, 33.6, 29.5, 29.5, 29.5, 26.6 |
| 4g | 8.32(d, 1H), 7.57(d, J=8.4 Hz, 2H), 7.44(d, J=8.4 Hz, 2H), 7.09(s, 1H), 4.44(dd, J=8.4, 4.8 Hz, 1H), 3.51—3.56(m, 2H), 3.15(q, J=4.8 Hz, 1H), 2.99(q, J=8.4 Hz, 1H), 2.50—2.55(m, 2H) | 175.5, 172.6, 168.8, 136.8, 132.2, 131.1, 129.0, 128.0, 128.0, 127.7, 127.7, 115.6, 53.0, 35.6, 34.5, 26.5 |
| 4h | 8.53(s, 1H, —COOH),8.20(d, J=7.8 Hz, 1H, imidazole NH)7.91(d, J=8.4Hz, 2H), 7.75(d, J=8.4 Hz, 2H), 7.73(s, 1H, —CONH), 7.72(s, 1H, —CONH), 7.55(s, 1H), 7.49(t, J=7.8 Hz, 2H), 7.41(d, J=7.8 Hz, 1H), 6.81(s, 1H), 4.44(d, J=5.4 Hz, 1H), 3.45(d, J=6.0 Hz, 2H), 2.95(q, J=5.4 Hz, 1H), 2.84(q, J=5.4 Hz, 1H), 2.41(q, J=7.2 Hz, 2H) | 173.1, 170.3, 165.8, 142.6, 142.6, 139.2, 134.7, 133.3, 133.3, 129.0, 129.0, 128.0, 127.8, 127.8, 126.8, 126.8, 126.4, 126.4, 52.3, 36.0, 35.2, 28.9 |
| 4i | 8.34(s, 1H), 7.31(t, J=7.2 Hz, 2H), 7.26(t, J=7.2 Hz, 1H), 7.21(d, J=7.2 Hz, 2H), 7.09(s, 1H), 4.36(q, J=5.4 Hz, 1H), 3.49(s, 2H), 3.34(t, J=6.0 Hz, 2H), 3.07(d, J=4.8 Hz, 1H), 2.94(d, J=7.8 Hz, 1H), 2.40(t, J=6.0 Hz, 2H) | 175.7, 173.8, 172.4, 134.1, 132.6, 129.4, 128.3, 128.3, 128.1, 128.1, 126.5, 116.0, 53.4, 41.5, 35.1, 34.3, 26.8 |
| 4j | 8.45(s, 1H), 7.27(t, J=7.2 Hz, 2H), 7.15—7.20(m, 4H), 4.38(q, J=5.4 Hz, 1H), 3.22(t, J=6.6 Hz, 2H), 3.17(q, J=5.4 Hz, 1H), 3.14(q, J=5.4 Hz, 1H), 2.98(q, J=7.8 Hz, 1H), 2.82(t, J=7.2 Hz, 2H), 2.44(t, J=7.2 Hz, 2H), 2.26(t, J=6.6 Hz, 2H) | 175.5, 174.9, 172.3, 139.7, 132.3, 129.0, 127.9, 127.9, 127.6, 127.6, 125.6, 115.8, 53.2, 36.5, 34.7, 34.1, 30.5, 26.6 |
| 4k | 8.49(d, J=1.2 Hz, 1H), 7.18(s, 1H), 4.43(dd, J=7.8, 5.4 Hz, 1H), 3.62(s, 1H), 3.34(q, J=6.6 Hz, 2H), 3.15—3.19(m, 2H), 3.12(d, J=6.6 Hz, 1H), 3.01(t, J=7.2 Hz, 1H), 2.41(q, 3H), 2.15(t, J=7.2 Hz, 2H), 1.92(t, J=7.2 Hz, 1H), 1.66(s, 1H), 1.50—1.56(m, 3H), 1.32(t, J=7.8 Hz, 2H) | 176.1, 175.5, 172.4, 132.4, 129.1, 115.9, 55.7, 53.3, 39.5, 37.3, 34.9, 34.6, 34.3, 32.9, 26.9, 26.7, 24.2 |
Table 2 1H NMR and 13C NMR for compounds 4a, 4c, 4e—4k
| Compd. | 1H NMR(D2O, 600 MHz), δ | 13C NMR(D2O, 150 MHz), δ |
|---|---|---|
| 4a | 8.45(s, 1H), 7.16(s, 1H), 4.41(dd, J=7.8, 4.8 Hz, 1H), 3.32(q, J=6.6 Hz, 2H), 3.16(q, J=4.8 Hz, 1H), 3.00(q, J=8.4 Hz, 1H), 2.40(t, J=6.6 Hz, 2H), 2.09(t, J=7.2 Hz, 2H), 1.47(q, J=7.2 Hz, 2H), 0.78(t, J=7.2 Hz, 3H) | 176.3, 175.6, 172.5, 132.5, 129.2, 115.9, 53.3, 36.8, 34.9, 34.3, 26.7, 18.1, 11.8 |
| 4c | 8.47(s, 1H), 7.17(s, 1H), 4.42(t, J=7.8, 5.4 Hz, 1H), 3.33(q, J=6.6 Hz, 2H), 3.17(q, J=5.4 Hz, 1H), 3.01(q, J=7.8 Hz, 1H), 2.41(t, J=6.6 Hz, 2H), 2.12(t, J=7.2 Hz, 2H), 1.47(s, 2H), 1.19(s, 10H), 0.78(t, J=7.2 Hz, 3H) | 176.5, 175.5, 172.4, 132.5, 129.2, 115.9, 53.3, 34.9, 34.9, 34.3, 30.2, 27.5, 27.4, 27.3, 26.7, 24.5, 21.2, 12.6 |
| 4e | 8.17(s, 1H), 7.52(d, J=7.8 Hz, 2H), 7.28(d, J=8.4 Hz, 2H), 7.05(s, 1H), 4.44(s, 1H), 3.54(q, J=6.0 Hz, 2H), 3.13(t, J=4.2 Hz, 1H), 2.98(q, J=8.4 Hz, 1H), 2.56(d, J=6.6 Hz, 1H), 2.50(d, J=6.6 Hz, 1H), 2.34(s, 1H) | 175.6, 172.7, 169.7, 142.5, 132.2, 129.6, 129.1, 128.6, 128.6, 126.2, 126.2, 115.5, 53.0, 35.5, 34.5, 26.5, 19.7 |
| 4f | 8.15(s, 1H), 7.59(d, J=8.4 Hz, 2H), 7.53(d, J=8.4 Hz, 2H), 7.06(s, 1H), 4.45(dd, J=8.4, 4.8 Hz, 1H), 3.52—3.58(m, 2H), 3.14(q, J=4.8 Hz, 1H), 2.97(q, J=8.4 Hz, 1H), 2.51—2.57(m, 2H), 1.26(s, 9H) | 175.6, 172.7, 169.5, 155.5, 132.1, 129.6, 129.1, 126.1, 126.1, 125.0, 125.0, 115.6, 53.0, 35.5, 34.5, 33.6, 29.5, 29.5, 29.5, 26.6 |
| 4g | 8.32(d, 1H), 7.57(d, J=8.4 Hz, 2H), 7.44(d, J=8.4 Hz, 2H), 7.09(s, 1H), 4.44(dd, J=8.4, 4.8 Hz, 1H), 3.51—3.56(m, 2H), 3.15(q, J=4.8 Hz, 1H), 2.99(q, J=8.4 Hz, 1H), 2.50—2.55(m, 2H) | 175.5, 172.6, 168.8, 136.8, 132.2, 131.1, 129.0, 128.0, 128.0, 127.7, 127.7, 115.6, 53.0, 35.6, 34.5, 26.5 |
| 4h | 8.53(s, 1H, —COOH),8.20(d, J=7.8 Hz, 1H, imidazole NH)7.91(d, J=8.4Hz, 2H), 7.75(d, J=8.4 Hz, 2H), 7.73(s, 1H, —CONH), 7.72(s, 1H, —CONH), 7.55(s, 1H), 7.49(t, J=7.8 Hz, 2H), 7.41(d, J=7.8 Hz, 1H), 6.81(s, 1H), 4.44(d, J=5.4 Hz, 1H), 3.45(d, J=6.0 Hz, 2H), 2.95(q, J=5.4 Hz, 1H), 2.84(q, J=5.4 Hz, 1H), 2.41(q, J=7.2 Hz, 2H) | 173.1, 170.3, 165.8, 142.6, 142.6, 139.2, 134.7, 133.3, 133.3, 129.0, 129.0, 128.0, 127.8, 127.8, 126.8, 126.8, 126.4, 126.4, 52.3, 36.0, 35.2, 28.9 |
| 4i | 8.34(s, 1H), 7.31(t, J=7.2 Hz, 2H), 7.26(t, J=7.2 Hz, 1H), 7.21(d, J=7.2 Hz, 2H), 7.09(s, 1H), 4.36(q, J=5.4 Hz, 1H), 3.49(s, 2H), 3.34(t, J=6.0 Hz, 2H), 3.07(d, J=4.8 Hz, 1H), 2.94(d, J=7.8 Hz, 1H), 2.40(t, J=6.0 Hz, 2H) | 175.7, 173.8, 172.4, 134.1, 132.6, 129.4, 128.3, 128.3, 128.1, 128.1, 126.5, 116.0, 53.4, 41.5, 35.1, 34.3, 26.8 |
| 4j | 8.45(s, 1H), 7.27(t, J=7.2 Hz, 2H), 7.15—7.20(m, 4H), 4.38(q, J=5.4 Hz, 1H), 3.22(t, J=6.6 Hz, 2H), 3.17(q, J=5.4 Hz, 1H), 3.14(q, J=5.4 Hz, 1H), 2.98(q, J=7.8 Hz, 1H), 2.82(t, J=7.2 Hz, 2H), 2.44(t, J=7.2 Hz, 2H), 2.26(t, J=6.6 Hz, 2H) | 175.5, 174.9, 172.3, 139.7, 132.3, 129.0, 127.9, 127.9, 127.6, 127.6, 125.6, 115.8, 53.2, 36.5, 34.7, 34.1, 30.5, 26.6 |
| 4k | 8.49(d, J=1.2 Hz, 1H), 7.18(s, 1H), 4.43(dd, J=7.8, 5.4 Hz, 1H), 3.62(s, 1H), 3.34(q, J=6.6 Hz, 2H), 3.15—3.19(m, 2H), 3.12(d, J=6.6 Hz, 1H), 3.01(t, J=7.2 Hz, 1H), 2.41(q, 3H), 2.15(t, J=7.2 Hz, 2H), 1.92(t, J=7.2 Hz, 1H), 1.66(s, 1H), 1.50—1.56(m, 3H), 1.32(t, J=7.8 Hz, 2H) | 176.1, 175.5, 172.4, 132.4, 129.1, 115.9, 55.7, 53.3, 39.5, 37.3, 34.9, 34.6, 34.3, 32.9, 26.9, 26.7, 24.2 |
| Compd. | Scavenging ratio(%) | Compd. | Scavenging ratio(%) | ||||
|---|---|---|---|---|---|---|---|
| 1 h | 2 h | 3 h | 1 h | 2 h | 3 h | ||
| 4a | 25.6±0.9 | 30.4±0.4 | 37.0±0.5 | 4h | 38.7±0.6 | 51.5±1.6 | 60.5±0.5 |
| 4b | 22.0±0.6 | 32.9±2.3 | 40.3±1.2 | 4i | 21.0±0.6 | 26.5±1.2 | 40.7±1.2 |
| 4c | 21.9±0.4 | 42.6±0.8 | 55.5±1.2 | 4j | 14.9±0.4 | 28.6±0.8 | 43.6±1.1 |
| 4d | 22.2±1.4 | 31.7±1.8 | 40.5±1.6 | 4k | 56.0±2.1 | 75.2±1.0 | 92.1±1.1 |
| 4e | 28.0±1.1 | 39.1±1.5 | 55.8±2.0 | Carnosine | 46.6±1.8 | 74.7±2.0 | 88.0±2.3 |
| 4f | 21.3±0.7 | 38.5±0.5 | 58.4±0.5 | β-Alanine | 12.3±1.5 | 27.6±1.7 | 41.1±2.0 |
| 4g | 22.9±1.4 | 39.7±1.2 | 58.2±0.4 | GABA | 10.4±0.8 | 23.4±0.4 | 39.0±2.3 |
Table 3 Acrolein scavenging activities of compounds 4a—4k
| Compd. | Scavenging ratio(%) | Compd. | Scavenging ratio(%) | ||||
|---|---|---|---|---|---|---|---|
| 1 h | 2 h | 3 h | 1 h | 2 h | 3 h | ||
| 4a | 25.6±0.9 | 30.4±0.4 | 37.0±0.5 | 4h | 38.7±0.6 | 51.5±1.6 | 60.5±0.5 |
| 4b | 22.0±0.6 | 32.9±2.3 | 40.3±1.2 | 4i | 21.0±0.6 | 26.5±1.2 | 40.7±1.2 |
| 4c | 21.9±0.4 | 42.6±0.8 | 55.5±1.2 | 4j | 14.9±0.4 | 28.6±0.8 | 43.6±1.1 |
| 4d | 22.2±1.4 | 31.7±1.8 | 40.5±1.6 | 4k | 56.0±2.1 | 75.2±1.0 | 92.1±1.1 |
| 4e | 28.0±1.1 | 39.1±1.5 | 55.8±2.0 | Carnosine | 46.6±1.8 | 74.7±2.0 | 88.0±2.3 |
| 4f | 21.3±0.7 | 38.5±0.5 | 58.4±0.5 | β-Alanine | 12.3±1.5 | 27.6±1.7 | 41.1±2.0 |
| 4g | 22.9±1.4 | 39.7±1.2 | 58.2±0.4 | GABA | 10.4±0.8 | 23.4±0.4 | 39.0±2.3 |
| Compd. | OD value* | Proliferation rate(%) | Compd. | OD value* | Proliferation rate(%) |
|---|---|---|---|---|---|
| 4a | 0.830±0.097 | 58.0 | 4h | 0.782±0.094 | 48.9 |
| 4b | 1.171±0.122 | 123.1 | 4i | 0.563±0.108 | 7.3 |
| 4c | 0.859±0.087 | 63.6 | 4j | 0.613±0.134 | 16.8 |
| 4d | 1.066±0.119 | 103.0 | 4k | 0.917±0.042 | 74.7 |
| 4e | 0.693±0.056 | 32.0 | Carnosine | 0.499±0.066 | -4.9 |
| 4f | 0.732±0.111 | 39.5 | Blank | 0.525±0.079 | 0 |
| 4g | 0.656±0.157 | 24.9 | ConA | 0.882±0.071 | 68.0 |
Table 4 Spleen cell proliferation activities of compounds 4a—4k
| Compd. | OD value* | Proliferation rate(%) | Compd. | OD value* | Proliferation rate(%) |
|---|---|---|---|---|---|
| 4a | 0.830±0.097 | 58.0 | 4h | 0.782±0.094 | 48.9 |
| 4b | 1.171±0.122 | 123.1 | 4i | 0.563±0.108 | 7.3 |
| 4c | 0.859±0.087 | 63.6 | 4j | 0.613±0.134 | 16.8 |
| 4d | 1.066±0.119 | 103.0 | 4k | 0.917±0.042 | 74.7 |
| 4e | 0.693±0.056 | 32.0 | Carnosine | 0.499±0.066 | -4.9 |
| 4f | 0.732±0.111 | 39.5 | Blank | 0.525±0.079 | 0 |
| 4g | 0.656±0.157 | 24.9 | ConA | 0.882±0.071 | 68.0 |
| Compd. | OD value | Proliferation rate(%) | Compd. | OD value | Proliferation rate(%) |
|---|---|---|---|---|---|
| 4a | 2.638±0.055 | 53.6 | 4h | 2.239±0.104 | 30.4 |
| 4b | 2.385±0.033 | 38.9 | 4i | 2.403±0.184 | 40.0 |
| 4c | 2.237±0.075 | 30.3 | 4j | 2.720±0.144 | 58.4 |
| 4d | 2.308±0.086 | 34.4 | 4k | 2.767±0.013 | 61.2 |
| 4e | 2.006±0.223 | 16.8 | Carnosine | 2.510±0.130 | 46.2 |
| 4f | 2.080±0.071 | 21.1 | Blank | 1.717±0.191 | 0 |
| 4g | 2.353±0.255 | 37.0 | H2O2 | 1.258±0.179 | -26.7 |
Table 5 ECV-304 cell protective effects of compounds 4a—4k
| Compd. | OD value | Proliferation rate(%) | Compd. | OD value | Proliferation rate(%) |
|---|---|---|---|---|---|
| 4a | 2.638±0.055 | 53.6 | 4h | 2.239±0.104 | 30.4 |
| 4b | 2.385±0.033 | 38.9 | 4i | 2.403±0.184 | 40.0 |
| 4c | 2.237±0.075 | 30.3 | 4j | 2.720±0.144 | 58.4 |
| 4d | 2.308±0.086 | 34.4 | 4k | 2.767±0.013 | 61.2 |
| 4e | 2.006±0.223 | 16.8 | Carnosine | 2.510±0.130 | 46.2 |
| 4f | 2.080±0.071 | 21.1 | Blank | 1.717±0.191 | 0 |
| 4g | 2.353±0.255 | 37.0 | H2O2 | 1.258±0.179 | -26.7 |
| Compd. | Plasma stability assay | |||
|---|---|---|---|---|
| 0.5 h | 1 h | 1.5 h | 2 h | |
| 4a | 99.7±0.1 | 99.3±0.1 | 99.1±0.1 | 99.0±0.1 |
| 4b | 99.6±0.1 | 99.4±0.1 | 99.1±0.1 | 98.7±0.1 |
| 4c | 99.5±0.1 | 99.1±0.1 | 98.9±0.1 | 98.7±0.1 |
| 4d | 99.7±0.1 | 99.6±0.1 | 99.4±0.1 | 99.3±0.1 |
| 4e | 99.9±0.1 | 99.3±0.1 | 99.0±0.1 | 98.6±0.1 |
| 4f | 99.7±0.1 | 99.6±0.1 | 99.3±0.1 | 99.0±0.1 |
| 4g | 99.7±0.1 | 99.4±0.1 | 99.1±0.1 | 98.8±0.1 |
| 4h | 99.9±0.1 | 99.6±0.1 | 99.3±0.1 | 99.1±0.1 |
| 4i | 99.8±0.1 | 99.1±0.1 | 98.4±0.1 | 98.0±0.1 |
| 4j | 99.9±0.1 | 99.6±0.1 | 99.4±0.1 | 99.2±0.1 |
| 4k | 99.7±0.1 | 99.5±0.1 | 99.3±0.1 | 99.0±0.1 |
Table 6 Plasma stability assay of compounds 4a—4k
| Compd. | Plasma stability assay | |||
|---|---|---|---|---|
| 0.5 h | 1 h | 1.5 h | 2 h | |
| 4a | 99.7±0.1 | 99.3±0.1 | 99.1±0.1 | 99.0±0.1 |
| 4b | 99.6±0.1 | 99.4±0.1 | 99.1±0.1 | 98.7±0.1 |
| 4c | 99.5±0.1 | 99.1±0.1 | 98.9±0.1 | 98.7±0.1 |
| 4d | 99.7±0.1 | 99.6±0.1 | 99.4±0.1 | 99.3±0.1 |
| 4e | 99.9±0.1 | 99.3±0.1 | 99.0±0.1 | 98.6±0.1 |
| 4f | 99.7±0.1 | 99.6±0.1 | 99.3±0.1 | 99.0±0.1 |
| 4g | 99.7±0.1 | 99.4±0.1 | 99.1±0.1 | 98.8±0.1 |
| 4h | 99.9±0.1 | 99.6±0.1 | 99.3±0.1 | 99.1±0.1 |
| 4i | 99.8±0.1 | 99.1±0.1 | 98.4±0.1 | 98.0±0.1 |
| 4j | 99.9±0.1 | 99.6±0.1 | 99.4±0.1 | 99.2±0.1 |
| 4k | 99.7±0.1 | 99.5±0.1 | 99.3±0.1 | 99.0±0.1 |
| [1] | Kohen R., Yamamoto Y., Cundy K. C., Ames B. N., Proc. Natl. Acad. Sci. USA,1988, 85(9), 3175—3179 |
| [2] | Hipkiss A. R., J. Alzheimers. Dis., 2007, 11(2), 229—240 |
| [3] | Guiotto A., Calderan A., Ruzza P., Borin G., Curr. Med. Chem., 2005, 12(20), 2293—2315 |
| [4] | Aldini G., Carini M., Beretta G., Bradamante S., Facino R. M., Biochem. Biophys. Res. Commun., 2002, 298(5), 699—706 |
| [5] | Carini M., Aldini G., Beretta G., Arlandini E., Facino R. M., J. Mass Spectrom., 2003, 38(9), 996—1006 |
| [6] | Aldini G., Facino R. M., Beretta G., Carini M., Biofactors,2005, 24(1—4), 77—87 |
| [7] | Hipkiss A. R., Michaelis J., Syrris P., FEBS. Lett., 1995, 371(1), 81—85 |
| [8] | Hobart L. J., Seibel I., Yeargans G. S., Seidler N. W., Life Sci., 2004, 75(11), 1379—1389 |
| [9] | Szwergold B. S., Biochem. Biophys. Res. Commun., 2005, 336(1), 36—41 |
| [10] | Nielsen C. U., Supuran C. T., Scozzafava A.,Frokjaer S., Steffansen B., Brodin B., Pharm. Res., 2002, 19(9), 1337—1344 |
| [11] | Stvolinsky S. L., Bulygina E. R., Fedorova T. N., Meguro K.,Sato T., Tyulina O. V., Abe H., Boldyrev A. A., Cell Mol. Neuro-biol., 2010, 30(3), 395—404 |
| [12] | Stvolinsky S., Antipin M., Meguro K., Sato T., Abe H., Boldyrev A., Rejuvenation Res., 2010, 13(4), 453—457 |
| [13] | Lanza V., Bellia F., D’Agata R., Grasso G., Rizzarelli E., Vecchio G., J. Inorg. Biochem., 2011, 105(2), 181—188 |
| [14] | Saada M. C., Montero J. L., Vullo D., Scozzafava A., Winum J. Y., Supuran C. T., J. Med. Chem., 2011, 54(5), 1170—1177 |
| [15] | Bertinaria M., Rolando B., Giorgis M., Montanaro G., Guglielmo S., Buonsanti M. F., Carabelli V., Gavello D., Daniele P. G., Fruttero R., Gasco A., J. Med. Chem., 2011, 54(2), 611—621 |
| [16] | Bertinaria M., Rolando B., Giorgis M., Montanaro G., Marini E., Collino M., Benetti E., Daniele P. G., Fruttero R., Gasco A., Eur. J. Med. Chem., 2012, 54, 103—112 |
| [17] | Bellia F., Oliveri V., Rizzarelli E., Vecchio G., Eur. J. Med. Chem., 2013, 70, 225—232 |
| [18] | Sun Q., Burke J. P., Phan J., Burns M. C., Olejniczak E. T., Waterson A. G., Lee T., Rossanese O. W., Fesik S. W., Angew. Chem. Int. Ed. Engl., 2012, 51(25), 6140—6143 |
| [19] | Okabe S., Sonehara T., Sato M., Mazaki M., Yamanaka T., Yoshioka M., Okai K., Kinoshita T., Aluminum Salts of N-acylcarnosine and Their Use DE 3034248, 1980-09-11 |
| [20] | Li Y. F., He R. R., Tsoi B., Li X. D., Li W. X., Abe K., Kurihara H., PLoS One, 2012, 7(4), e33190-1—e33190-11 |
| [21] | Wang Y. K., Hong Y. J., Wei M., Wu Y., Huang Z. Q., Chen R. Z., Chen H. Z., J. Ethnopharmacol., 2010, 132(1), 233—239 |
| [22] | Yu M. H., Du F. Y., Rao X., Yao F. L., Yang J., Chem. J. Chinese Universities, 2013, 34(3), 746—750 |
| (于美华, 杜凤移, 饶霞, 姚芳莲, 杨军. 高等学校化学学报, 2013, 34(3), 746—750) |
| [1] | ZHANG Luyun, XU Qian, XIA Guangqing, CONG Li, WANG Yu, ZHANG Hui, ZHU Junyi, ZANG Hao. Synthesis and Biological Activity of Lipoic Acid Ester Derivatives† [J]. Chem. J. Chinese Universities, 2018, 39(10): 2198. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||